AP Chemistry 8.11 pH and Solubility Study Notes - New Syllabus Effective fall 2024
AP Chemistry 8.11 pH and Solubility Study Notes- New syllabus
AP Chemistry 8.11 pH and Solubility Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Identify the qualitative effect of changes in pH on the solubility of a salt.
Key Concepts:
- Qualitative Effect of pH on the Solubility of a Salt
Qualitative Effect of pH on the Solubility of a Salt
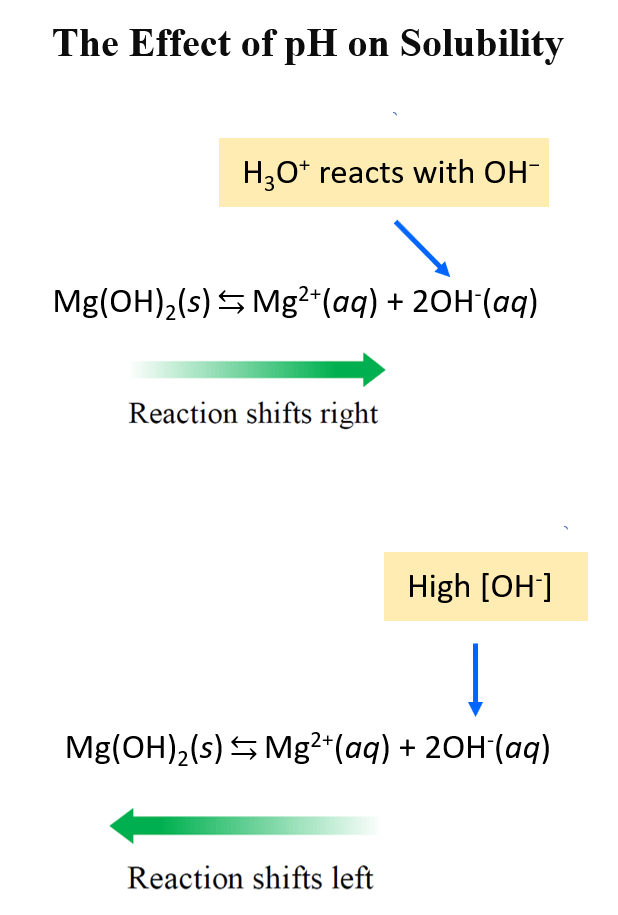
The solubility of an ionic compound can depend on the pH of the solution when one of its ions acts as a weak acid, a weak base, or the hydroxide ion. Changes in pH shift the solubility equilibrium according to Le Châtelier’s Principle.
General Principle: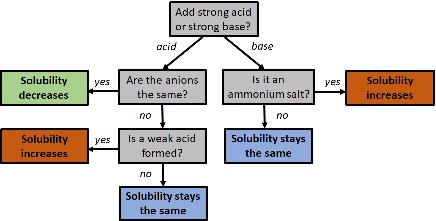
- When a solution becomes more acidic (lower pH), the concentration of \( \mathrm{H^+} \) ions increases.
- When a solution becomes more basic (higher pH), the concentration of \( \mathrm{OH^-} \) ions increases.
- If an ion of the salt reacts with \( \mathrm{H^+} \) or \( \mathrm{OH^-} \), the equilibrium shifts to dissolve more or less solid, changing solubility.
Key Relationships:
- Salts with basic anions (e.g., \( \mathrm{CO_3^{2-}} \), \( \mathrm{OH^-} \), \( \mathrm{S^{2-}} \), \( \mathrm{CH_3COO^-} \)) become more soluble in acidic solutions, because \( \mathrm{H^+} \) ions react with the anion to form weak acids, removing the anion from solution and driving dissolution forward.
- Salts with acidic cations (e.g., \( \mathrm{Fe^{3+}} \), \( \mathrm{Al^{3+}} \), \( \mathrm{NH_4^+} \)) become more soluble in basic solutions, because \( \mathrm{OH^-} \) reacts with the cation to form hydroxide complexes or neutral species.

- Salts of strong acids and strong bases (e.g., \( \mathrm{NaCl} \), \( \mathrm{KNO_3} \)) have no pH-dependent solubility.
Conceptual Explanation Using Le Châtelier’s Principle:
For a sparingly soluble salt \( \mathrm{AB} \):
\( \mathrm{AB(s) \rightleftharpoons A^+(aq) + B^-(aq)} \)
- If \( \mathrm{B^-} \) reacts with \( \mathrm{H^+} \) to form \( \mathrm{HB} \), the \( \mathrm{B^-} \) concentration decreases.
- Le Châtelier’s Principle shifts equilibrium to the right → more \( \mathrm{AB} \) dissolves → solubility increases.
- Conversely, adding \( \mathrm{OH^-} \) suppresses \( \mathrm{B^-} \) protonation, decreasing solubility.
| Type of Ion in Salt | Effect of Decreasing pH | Effect of Increasing pH |
|---|---|---|
| Basic anion (e.g., \( \mathrm{CO_3^{2-}} \), \( \mathrm{S^{2-}} \)) | Solubility ↑ (acid reacts with base anion) | Solubility ↓ |
| Acidic cation (e.g., \( \mathrm{Fe^{3+}} \), \( \mathrm{NH_4^+} \)) | Solubility ↓ | Solubility ↑ (base reacts with cation) |
| Neutral ions (e.g., \( \mathrm{Na^+} \), \( \mathrm{Cl^-} \)) | No effect | No effect |
Example :
Explain qualitatively how the solubility of \( \mathrm{CaCO_3} \) changes when acid is added to the solution.
▶️ Answer / Explanation
Step 1: Write the dissolution equilibrium:
\( \mathrm{CaCO_3(s) \rightleftharpoons Ca^{2+}(aq) + CO_3^{2-}(aq)} \)
Step 2: \( \mathrm{CO_3^{2-}} \) is a basic anion that reacts with \( \mathrm{H^+} \):
\( \mathrm{CO_3^{2-} + 2H^+ \rightarrow H_2CO_3 \rightarrow CO_2(g) + H_2O(l)} \)
Step 3: The reaction removes \( \mathrm{CO_3^{2-}} \) from solution, shifting equilibrium right and dissolving more \( \mathrm{CaCO_3} \).
Final Answer: The solubility of \( \mathrm{CaCO_3} \) increases as pH decreases (acidic conditions).
Example :
How does increasing pH affect the solubility of \( \mathrm{NH_4Cl} \)?
▶️ Answer / Explanation
Step 1: Write the dissociation equilibrium:
\( \mathrm{NH_4Cl(s) \rightleftharpoons NH_4^+(aq) + Cl^-(aq)} \)
Step 2: \( \mathrm{NH_4^+} \) is a weak acid that reacts with \( \mathrm{OH^-} \):
\( \mathrm{NH_4^+(aq) + OH^-(aq) \rightarrow NH_3(aq) + H_2O(l)} \)
Step 3: The reaction removes \( \mathrm{NH_4^+} \) from solution, shifting equilibrium to the right → more \( \mathrm{NH_4Cl} \) dissolves.
Final Answer: Increasing pH (more basic) increases the solubility of \( \mathrm{NH_4Cl} \).