AP Chemistry 9.6 Free Energy of Dissolution Study Notes - New Syllabus Effective fall 2024
AP Chemistry 9.6 Free Energy of Dissolution Study Notes- New syllabus
AP Chemistry 9.6 Free Energy of Dissolution Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship between the solubility of a salt and changes in the enthalpy and entropy that occur in the dissolution process.
Key Concepts:
- Relationship Between Solubility, Enthalpy, and Entropy in Dissolution Processes
Relationship Between Solubility, Enthalpy, and Entropy in Dissolution Processes
The solubility of a salt in a solvent depends on the overall free energy change (\( \mathrm{\Delta G^\circ_{dissolution}} \)) associated with the process of dissolving the solid into its ions or molecules. This free energy change arises from three major energetic and entropic contributions that occur during dissolution: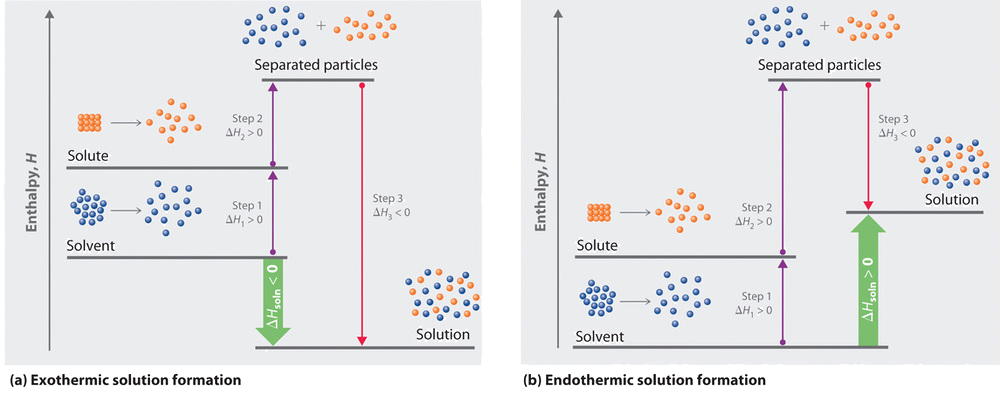
- Breaking solute–solute interactions (in the solid crystal)
- Disrupting solvent–solvent interactions (among solvent molecules)
- Forming solute–solvent interactions (hydration or solvation)
The overall process can be summarized as:
\( \mathrm{\text{solute (s)} + \text{solvent (l)} \rightarrow \text{solute-solvent (aq)}} \)
Key Thermodynamic Relationship
\( \mathrm{\Delta G^\circ_{dissolution} = \Delta H^\circ_{dissolution} – T \Delta S^\circ_{dissolution}} \)
- \( \mathrm{\Delta H^\circ_{dissolution}} \): Enthalpy change due to bond breaking/forming during dissolution.
- \( \mathrm{\Delta S^\circ_{dissolution}} \): Entropy change due to disorder increase or decrease.
- \( \mathrm{\Delta G^\circ_{dissolution}} \): Determines if dissolution is thermodynamically favored (\( \mathrm{\Delta G^\circ < 0} \)).
Stepwise Contributions During Dissolution
| Step | Process | Effect on Enthalpy (ΔH°) | Effect on Entropy (ΔS°) |
|---|---|---|---|
| 1 | Breaking solute–solute interactions (ionic bonds in salt) | Endothermic (+ΔH°) | Slight increase (+ΔS°) |
| 2 | Breaking solvent–solvent interactions (e.g., H-bonds in water) | Endothermic (+ΔH°) | Slight increase (+ΔS°) |
| 3 | Forming solute–solvent interactions (hydration/solvation) | Exothermic (−ΔH°) | Decrease in randomness (−ΔS°) |
Net Result: The total dissolution enthalpy and entropy depend on the relative magnitudes of these three steps. They may partially cancel each other, making \( \mathrm{\Delta G^\circ_{dissolution}} \) difficult to predict exactly.
Concept Summary
- If \( \mathrm{\Delta G^\circ_{dissolution} < 0} \), dissolution is spontaneous and the salt is soluble.
- If \( \mathrm{\Delta G^\circ_{dissolution} > 0} \), dissolution is nonspontaneous and the salt is insoluble or sparingly soluble.
- Often, solubility increases with temperature for endothermic dissolutions and decreases with temperature for exothermic dissolutions (Le Châtelier’s principle).
Key Idea:
The solubility of a salt depends on both the enthalpy change and entropy change of dissolution. If the increase in entropy (\( \mathrm{+T\Delta S^\circ} \)) outweighs any endothermic enthalpy contribution, the process is spontaneous and the salt is soluble. Predicting total \( \mathrm{\Delta G^\circ} \) can be complex because enthalpic and entropic contributions from multiple steps often partially cancel.
Example:
Explain why sodium chloride (\( \mathrm{NaCl} \)) dissolves in water even though its dissolution process is slightly endothermic (\( \mathrm{\Delta H^\circ_{dissolution} = +3.9\ kJ/mol} \)).
▶️ Answer / Explanation
Step 1: Write the dissolution process:
\( \mathrm{NaCl(s) \rightarrow Na^+(aq) + Cl^-(aq)} \)
Step 2: Energy changes involved:
- Breaking ionic lattice (Na⁺–Cl⁻): endothermic (+ΔH°)
- Hydration of ions by water molecules: exothermic (−ΔH°)
Step 3: Entropy change:
When NaCl dissolves, ions become dispersed and mobile → disorder increases → \( \mathrm{+ΔS°} \).
Step 4: Free energy change:
\( \mathrm{\Delta G^\circ = \Delta H^\circ – T\Delta S^\circ} \)
Even though \( \mathrm{\Delta H^\circ} \) is slightly positive, \( \mathrm{T\Delta S^\circ} \) is positive and large enough to make \( \mathrm{\Delta G^\circ < 0} \).
Step 5: Interpretation:
- Dissolution is entropy-driven (spontaneous due to increased disorder).
- \( \mathrm{\Delta G^\circ < 0} \): process is thermodynamically favored → NaCl is soluble.
Final Answer: \( \mathrm{NaCl} \) dissolves spontaneously because the positive entropy change (\( \mathrm{\Delta S^\circ} \)) outweighs the slightly endothermic enthalpy change, resulting in \( \mathrm{\Delta G^\circ < 0} \).
Example:
The dissolution of \( \mathrm{NH_4NO_3(s)} \) in water is strongly endothermic (\( \mathrm{\Delta H^\circ_{dissolution} = +25.7\ kJ/mol} \)). Yet, it occurs spontaneously and is used in cold packs. Explain why.
▶️ Answer / Explanation
Step 1: For dissolution:
\( \mathrm{NH_4NO_3(s) \rightarrow NH_4^+(aq) + NO_3^-(aq)} \)
Step 2: The large positive \( \mathrm{\Delta H^\circ} \) means heat is absorbed from surroundings → cooling effect.
Step 3: The entropy change \( \mathrm{(+\Delta S^\circ)} \) is significant because ions disperse in solution → much greater disorder.
Step 4: \( \mathrm{\Delta G^\circ = \Delta H^\circ – T\Delta S^\circ} \)
At room temperature, the \( \mathrm{T\Delta S^\circ} \) term is large enough to overcome the positive \( \mathrm{\Delta H^\circ} \), leading to \( \mathrm{\Delta G^\circ < 0} \).
Step 5: Interpretation:
- The process is entropy-driven.
- Even though it absorbs heat, the increase in disorder makes it spontaneous.
Final Answer: The dissolution of \( \mathrm{NH_4NO_3} \) is spontaneous (\( \mathrm{\Delta G^\circ < 0} \)) because it is highly entropy-favored despite being endothermic.
