AP Chemistry 5.7 Introduction to Reaction Mechanisms Study Notes - New Syllabus Effective fall 2024
AP Chemistry 5.7 Introduction to Reaction Mechanisms Study Notes- New syllabus
AP Chemistry 5.7 Introduction to Reaction Mechanisms Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Identify the components of a reaction mechanism.
Key Concepts:
- Reaction Mechanisms and Elementary Steps
- Consistency of Elementary Steps with the Overall Balanced Equation
- Reaction Intermediates and Their Role
- Experimental Detection of Reaction Intermediates
Reaction Mechanisms and Elementary Steps
A reaction mechanism describes the detailed sequence of elementary reactions (steps) that together make up an overall chemical reaction. Each elementary step shows a specific molecular event such as a collision between reactants or the formation of an intermediate — that contributes to the overall transformation.
Components of a Reaction Mechanism: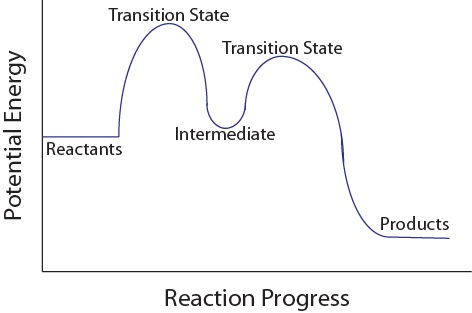
- Reactants: Starting substances that participate in the reaction.
- Products: Final substances formed at the end of the mechanism.
- Intermediates: Species produced in one step and consumed in another (temporary, do not appear in the net equation).
- Catalysts: Species that appear in the mechanism but are regenerated at the end (not consumed overall).
The complete reaction mechanism gives a microscopic view of how reactants are converted to products through a sequence of simpler collisions and rearrangements.
Example — Reaction Mechanism Representation
Overall reaction:
\( \mathrm{2NO_2 \rightarrow 2NO + O_2} \)
Possible mechanism:
- \( \mathrm{NO_2 \rightarrow NO + O} \)
- \( \mathrm{O + NO_2 \rightarrow NO + O_2} \)
Explanation:
- Step 1 produces an oxygen atom intermediate (\( \mathrm{O} \)).
- Step 2 consumes that oxygen atom, forming \( \mathrm{O_2} \).
- When both steps are added, intermediates cancel out, and the mechanism reproduces the overall balanced reaction.
Example
For the following mechanism, identify the reactant(s), product(s), intermediate(s), and catalyst (if any):
Step 1: \( \mathrm{Cl_2 \rightarrow 2Cl} \)
Step 2: \( \mathrm{Cl + CH_4 \rightarrow CH_3 + HCl} \)
Step 3: \( \mathrm{CH_3 + Cl_2 \rightarrow CH_3Cl + Cl} \)
▶️ Answer / Explanation
Step 1: Identify each component:
- Reactants: \( \mathrm{Cl_2, CH_4} \)
- Products: \( \mathrm{CH_3Cl, HCl} \)
- Intermediates: \( \mathrm{Cl, CH_3} \) (formed and later consumed)
- Catalyst: None (no species appears at both start and end unchanged)
Step 2: Confirm that the mechanism leads to the overall equation:
\( \mathrm{CH_4 + Cl_2 \rightarrow CH_3Cl + HCl} \)
Result: The species \( \mathrm{Cl} \) and \( \mathrm{CH_3} \) act as intermediates, appearing only during the reaction process. The mechanism explains how bonds are broken and formed step by step.
Consistency of Elementary Steps with the Overall Balanced Equation
Each reaction mechanism is composed of several elementary steps, each representing a simple molecular event such as a collision or rearrangement. When all of these steps are combined, their sum must exactly reproduce the overall balanced chemical equation — with the same reactants, products, and stoichiometric coefficients.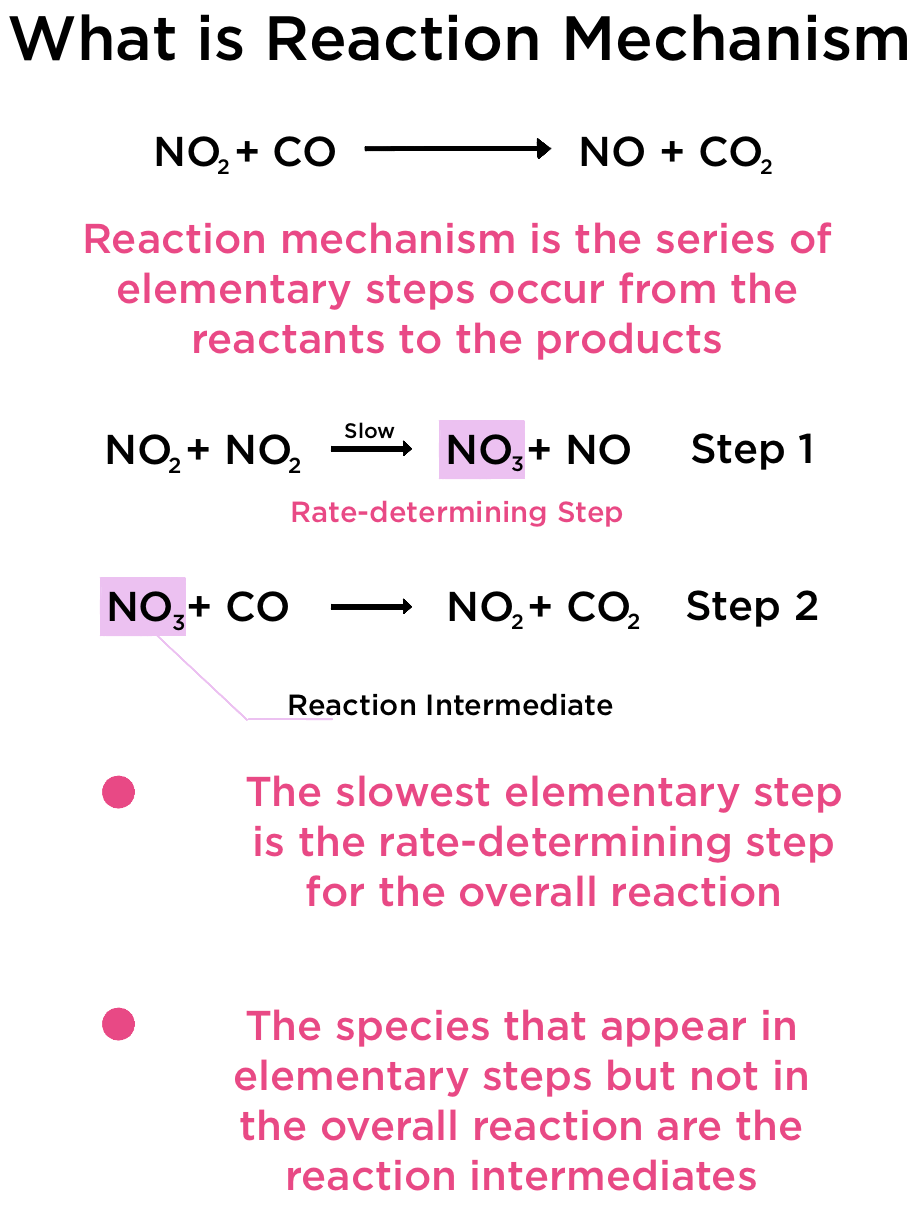
- The individual elementary steps describe what happens at the molecular level.
- When all steps are added together, the resulting equation must match the experimentally determined overall reaction.
- Species that are both produced and consumed in different steps are called intermediates and cancel out in the overall equation.
Mathematical Representation:
\( \mathrm{\sum (elementary\ steps) = overall\ balanced\ reaction} \)
Only species that remain after cancellation appear in the final balanced equation these are the reactants and products.
Key Idea: A valid mechanism must satisfy two essential conditions:
- The sum of the elementary steps reproduces the overall balanced reaction.
- The mechanism’s predicted rate law must agree with the experimentally determined rate law.
Example
The overall reaction is:
\( \mathrm{2NO + O_2 \rightarrow 2NO_2} \)
A proposed mechanism is:
- \( \mathrm{NO + O_2 \rightarrow NO_3} \)
- \( \mathrm{NO_3 + NO \rightarrow 2NO_2} \)
Show that this mechanism is consistent with the overall reaction.
▶️ Answer / Explanation
Step 1: Add the two steps together:
\( \mathrm{NO + O_2 + NO_3 + NO \rightarrow NO_3 + 2NO_2} \)
Step 2: Cancel any intermediates that appear on both sides.
\( \mathrm{NO_3} \) appears on both sides, so it cancels.
Step 3: Simplify the result:
\( \mathrm{2NO + O_2 \rightarrow 2NO_2} \)
Step 4: Interpretation:
- The overall reaction matches the experimentally determined equation.
- The species \( \mathrm{NO_3} \) is an intermediate — it forms in Step 1 and is used in Step 2.
Result: Since the sum of elementary steps reproduces the overall equation, the mechanism is chemically consistent.
Reaction Intermediates and Their Role
A reaction intermediate is a species that is produced in one elementary step of a reaction mechanism and consumed in a subsequent step. It exists only temporarily during the course of the reaction and does not appear in the overall balanced chemical equation.
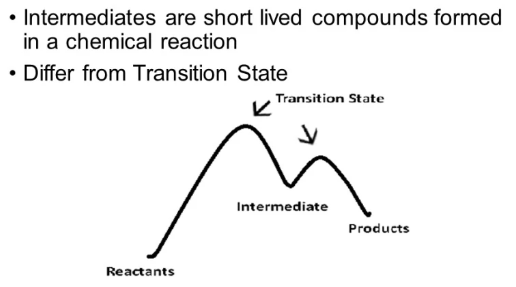
Key Characteristics of Reaction Intermediates:
- They are formed and then used up — never accumulate in measurable amounts.
- They are often unstable and exist only momentarily.
- They do not appear in the overall reaction since they cancel out when steps are added.
- They may sometimes be detected experimentally through spectroscopic or kinetic methods, providing evidence for the mechanism.
Distinguishing Intermediates from Catalysts:
| Property | Intermediate | Catalyst |
|---|---|---|
| When it appears | Produced in one step, consumed later | Appears in the first step, regenerated in the last |
| Presence in overall equation | Cancels out (not shown) | Cancels out (not shown) |
| Function | Temporary intermediate species | Speeds up reaction by providing alternate pathway |
| Stability | Highly unstable and short-lived | Usually stable and reusable |
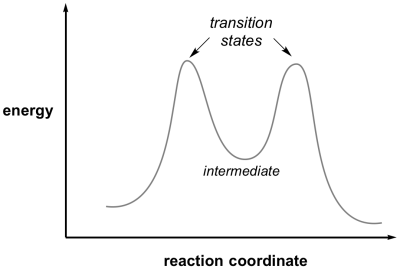
Intermediates are crucial in understanding reaction mechanisms because they explain how multi-step reactions proceed through a sequence of microscopic events rather than a single overall process.
Example
The following two-step mechanism is proposed for the oxidation of nitric oxide (\( \mathrm{NO} \)) to nitrogen dioxide (\( \mathrm{NO_2} \)):
Step 1: \( \mathrm{NO + O_2 \rightarrow NO_3} \) Step 2: \( \mathrm{NO_3 + NO \rightarrow 2NO_2} \)
Identify the intermediate and explain its role in the mechanism.
▶️ Answer / Explanation
Step 1: Determine species formed and consumed.
- \( \mathrm{NO_3} \) is formed in Step 1.
- \( \mathrm{NO_3} \) is consumed in Step 2.
Step 2: Check if it appears in the overall reaction.
Overall: \( \mathrm{2NO + O_2 \rightarrow 2NO_2} \)
\( \mathrm{NO_3} \) cancels out — it does not appear in the overall equation.
Step 3: Identify its role.
\( \mathrm{NO_3} \) acts as an intermediate that helps transfer oxygen between molecules of \( \mathrm{NO} \).
Result: The reaction intermediate is \( \mathrm{NO_3} \). It exists briefly during the reaction and provides a pathway for \( \mathrm{O_2} \) to oxidize \( \mathrm{NO} \) into \( \mathrm{NO_2.} \)
Experimental Detection of Reaction Intermediates
The experimental detection of a reaction intermediate provides crucial evidence in support of a proposed reaction mechanism. Because intermediates exist only temporarily, identifying them experimentally helps confirm that a mechanism correctly describes the sequence of molecular events leading from reactants to products.
- A valid reaction mechanism should predict the formation of certain intermediates.
- If these intermediates can be experimentally observed or detected, it supports the correctness of the proposed mechanism.
- Detection methods often involve sensitive spectroscopic or kinetic techniques capable of observing very short-lived species.
Common Experimental Techniques for Detecting Intermediates:
| Technique | Principle | Example of Application |
|---|---|---|
| Spectroscopy (UV-Vis, IR, or Mass) | Detects characteristic absorption or emission patterns of short-lived species. | Detection of \( \mathrm{NO_3} \) radicals in the oxidation of \( \mathrm{NO} \). |
| Stopped-Flow Techniques | Monitors very fast reactions by rapidly mixing reactants and measuring concentration changes over milliseconds. | Observation of colored intermediates during enzyme-catalyzed reactions. |
| Flash Photolysis | Uses a burst of light to generate reactive intermediates, then observes their decay spectroscopically. | Used to detect chlorine radicals in the photodissociation of \( \mathrm{Cl_2} \). |
| Mass Spectrometry | Identifies transient species based on their mass-to-charge ratio. | Detection of carbocation intermediates in hydrocarbon reactions. |
If an intermediate predicted by a mechanism is experimentally detected, it provides direct evidence that supports the proposed mechanism. Conversely, if no evidence of the predicted intermediate is found, the mechanism may need revision.
Quick Summary:
| Purpose | Experimental Methods | Evidence Provided |
|---|---|---|
| Confirm mechanism steps | Spectroscopy, stopped-flow, flash photolysis | Detection of short-lived intermediates |
| Identify short-lived species | Mass spectrometry or optical methods | Direct proof of intermediate existence |
| Support kinetic models | Rate law and concentration measurements | Shows transient concentration changes match predictions |
Final Takeaway: Detecting a reaction intermediate experimentally provides strong evidence that a proposed reaction mechanism is correct. Such detection links theoretical mechanisms with observable chemical behavior, confirming how molecules transform step by step.
Example
The reaction between \( \mathrm{NO} \) and \( \mathrm{O_2} \) to form \( \mathrm{NO_2} \) is proposed to proceed through the intermediate \( \mathrm{NO_3} \):
Step 1: \( \mathrm{NO + O_2 \rightarrow NO_3} \) Step 2: \( \mathrm{NO_3 + NO \rightarrow 2NO_2} \)
How can experimental evidence be used to confirm that \( \mathrm{NO_3} \) is indeed a reaction intermediate?
▶️ Answer / Explanation
Step 1: Predict the intermediate from the mechanism — \( \mathrm{NO_3} \).
Step 2: Look for experimental detection.
Using UV-visible spectroscopy, a distinct absorption band corresponding to \( \mathrm{NO_3} \) is observed during the reaction.
Step 3: Interpret the result.
Since \( \mathrm{NO_3} \) is transiently detected and then disappears as \( \mathrm{NO_2} \) forms, this confirms its presence as an intermediate rather than as a product.
Result: Experimental detection of \( \mathrm{NO_3} \) supports the proposed two-step mechanism, providing strong evidence that the reaction proceeds through this intermediate.
