AP Chemistry 3.7 Solutions and Mixtures Notes - New Syllabus 2024-2025
AP Chemistry 3.7 Solutions and Mixtures Notes- New syllabus
AP Chemistry 3.7 Solutions and Mixtures Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Calculate the number of solute particles, volume, or molarity of solutions.
Key Concepts:
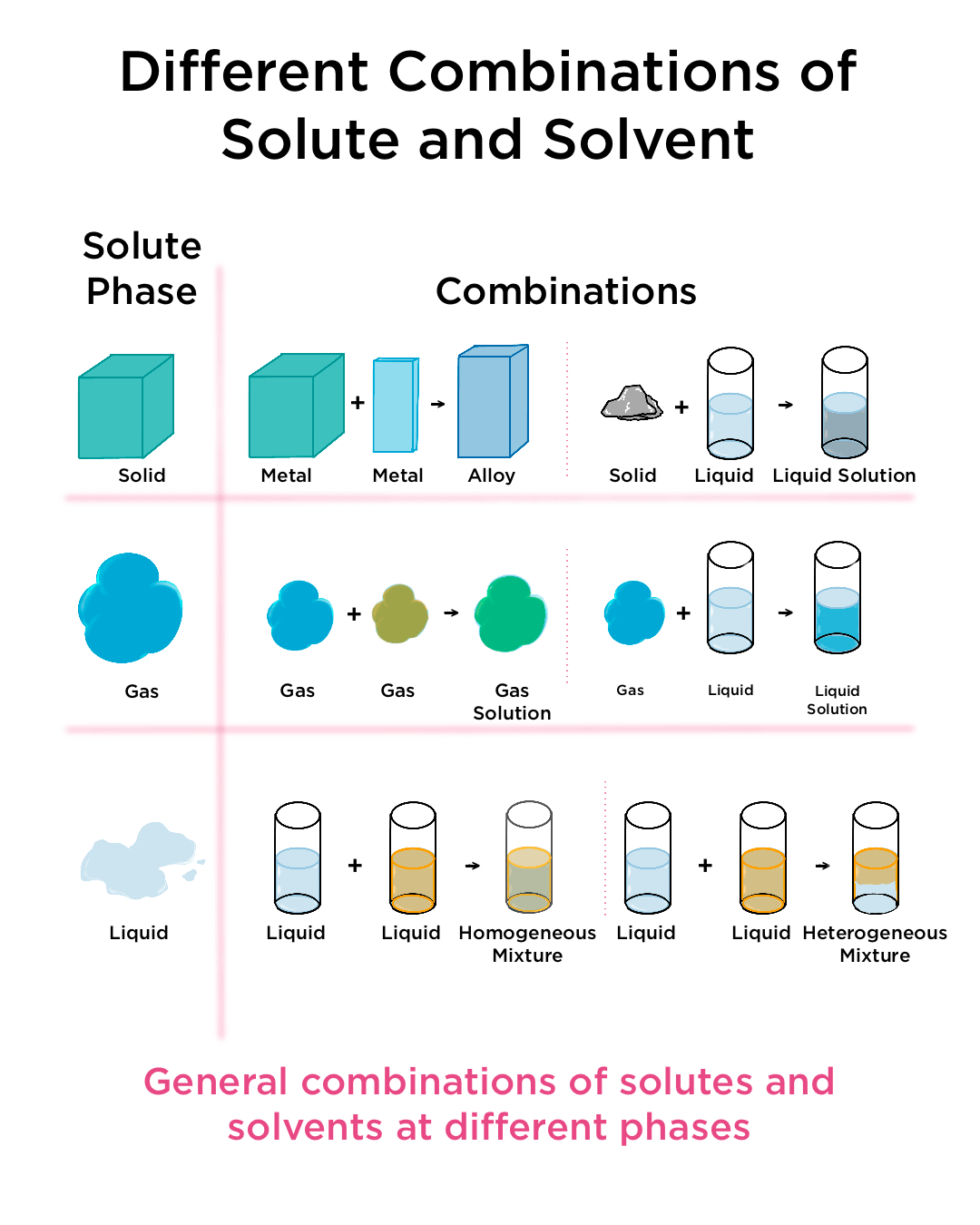
- Definition and Nature of Solutions vs. Heterogeneous Mixtures
- Expression of Solution Concentration (Molarity and Other Units)
Definition and Nature of Solutions vs. Heterogeneous Mixtures
A solution (also called a homogeneous mixture) is a uniform mixture of two or more substances in a single phase, where the macroscopic properties (such as composition, color, or density) are the same throughout the sample. In contrast, a heterogeneous mixture consists of visibly distinct components or phases, and its properties vary from one region to another.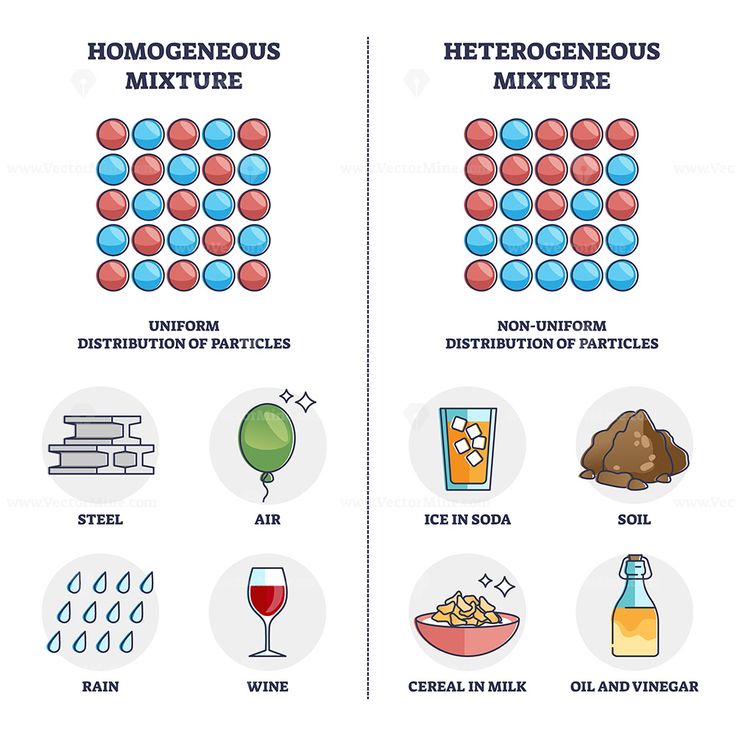
Key Properties:
1. Homogeneous Nature of Solutions:
- Solutions are uniform throughout—the composition is identical in every portion of the sample.
- Particles of the solute are evenly distributed at the molecular or ionic level in the solvent.
- Examples include salt water, air, and metal alloys such as brass.
2. Phases of Solutions:
- Gaseous solutions: Example—air (a mixture of nitrogen, oxygen, and other gases).
- Liquid solutions: Example—salt dissolved in water.
- Solid solutions: Example—alloys such as copper–zinc (brass) or iron–carbon (steel).

3. Macroscopic Uniformity:
- In a homogeneous solution, physical properties (such as density, color, and refractive index) do not vary with location in the sample.
- In a heterogeneous mixture, these properties do vary depending on where the measurement is made (e.g., sand at the bottom of water vs. clear liquid above).
4. Particle Size and Separation:
- In true solutions, the solute particles are at the molecular or ionic scale (diameter < 1 nm).
- They do not scatter light (no Tyndall effect) and cannot be separated by filtration.
- Heterogeneous mixtures, by contrast, have larger particles that can often be separated mechanically (e.g., filtration or decantation).
A solution is uniform at the molecular level, exhibiting consistent macroscopic properties throughout the sample. Heterogeneous mixtures, on the other hand, contain visibly or microscopically distinct regions with different compositions and properties.
Example :
Identify whether each of the following is a homogeneous or heterogeneous mixture, and justify your answer:
(a) salt water,
(b) oil and water,
(c) air,
(d) granite.
▶️ Answer/Explanation
Step 1: Evaluate uniformity and phase distribution.
(a) Salt water: Homogeneous — salt ions are uniformly distributed in water; composition is constant throughout.
(b) Oil and water: Heterogeneous — separate liquid phases form; properties differ between layers.
(c) Air: Homogeneous — uniform mixture of gases (mainly \(\mathrm{N_2}\) and \(\mathrm{O_2}\)); same composition everywhere.
(d) Granite: Heterogeneous — visible mineral grains of different colors and compositions.
Expression of Solution Concentration (Molarity and Other Units)
The composition of a solution can be expressed quantitatively in several ways that describe the relative amounts of solute and solvent present. The most common unit used in laboratory chemistry is molarity (M), which expresses the number of moles of solute per liter of solution.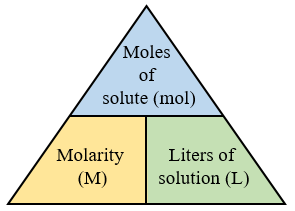
Key Formula:
\( \mathrm{M = \dfrac{n_{solute}}{V_{solution}}} \)
- \( \mathrm{M} \): Molarity (mol/L)
- \( \mathrm{n_{solute}} \): Moles of solute
- \( \mathrm{V_{solution}} \): Volume of solution in liters
Key Properties:
- Molarity expresses concentration as moles of solute per liter of total solution (not per liter of solvent). It provides a direct link between volume measurements and chemical amounts in moles, making it especially useful in stoichiometric calculations.
- Molarity (M) is measured in mol·L⁻¹ or mol/L. For example, a 1.00 M NaCl solution contains 1.00 mole of NaCl in each liter of solution.
- Because molarity depends on total solution volume, it can change with temperature (since liquid volumes expand or contract). Therefore, precise molarity measurements are temperature-specific.
Common Units for Solution Concentration
| Concentration Unit | Definition / Formula | Typical Use |
|---|---|---|
| Molarity (M) | \( \mathrm{M = \dfrac{n_{solute}}{V_{solution}}} \) | Common in lab reactions, titrations, and stoichiometry |
| Molality (m) | \( \mathrm{m = \dfrac{n_{solute}}{mass_{solvent\,(kg)}}} \) | Used when temperature variations matter (e.g., colligative properties) |
| Mass Percent | \( \mathrm{\%\,mass = \dfrac{mass_{solute}}{mass_{solution}} \times 100} \) | Used in industrial and food chemistry |
| Mole Fraction (\(X\)) | \( \mathrm{X_A = \dfrac{n_A}{n_{total}}} \) | Used in gas mixtures and vapor pressure laws |
| ppm / ppb | \( \mathrm{ppm = \dfrac{mass_{solute}}{mass_{solution}} \times 10^6} \) | Used for trace concentrations in environmental chemistry |
Example :
Calculate the molarity of a solution prepared by dissolving 5.85 g of sodium chloride (\(\mathrm{NaCl}\)) in enough water to make 0.500 L of solution.
▶️ Answer/Explanation
Step 1: Calculate moles of solute.
\( \mathrm{n = \dfrac{mass}{molar\ mass} = \dfrac{5.85\,g}{58.44\,g/mol} = 0.100\,mol.} \)
Step 2: Apply molarity formula.
\( \mathrm{M = \dfrac{n}{V} = \dfrac{0.100}{0.500} = 0.200\,M.} \)
Final Answer: The solution concentration is \( \mathrm{0.200\,M\,NaCl} \).