AP Chemistry 4.3 Representations of Reactions Study Notes - New Syllabus Effective fall 2024
AP Chemistry 4.3 Representations of Reactions Study Notes- New syllabus
AP Chemistry 4.3 Representations of Reactions Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Represent a given chemical reaction or physical process with a consistent particulate model.
Key Concepts:
- Particulate Representation of Chemical and Physical Processes
Particulate Representation of Chemical and Physical Processes
A particulate model is a visual or conceptual representation that illustrates how atoms, ions, or molecules interact and rearrange during a chemical or physical process. Balanced chemical equations can be directly translated into such particulate-level representations to show the rearrangement of matter while maintaining the conservation of mass and charge.
1. Purpose of Particulate Representations: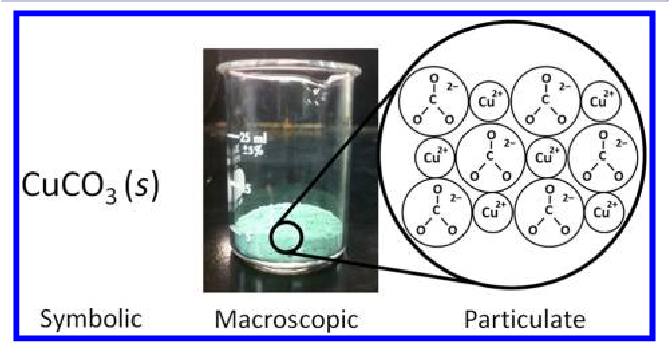
- To visualize the microscopic behavior of particles that underlies a chemical or physical change.
- To connect symbolic equations (macroscopic scale) with atomic-level interactions (microscopic scale).
- To demonstrate that atoms are conserved during a reaction, even as bonds are broken and reformed.
2. Principles of Particulate Models:
- Conservation of Matter: The same number of each type of atom appears before and after the reaction — none are lost or created.
- Conservation of Charge: The total electrical charge of the system remains constant throughout the process.
- Stoichiometric Ratios: The relative numbers of reactant and product particles correspond to the coefficients in the balanced chemical equation.
- Phase Representation:
- Solids — closely packed particles.
- Liquids — particles in close contact but randomly arranged.
- Gases — widely spaced particles moving freely.
- Aqueous ions — free ions surrounded by solvent (usually water) molecules.
3. Translating Symbolic Equations to Particulate Models:
Each particle or ion shown in the diagram represents a molecule or formula unit. The ratio of particles must reflect the coefficients in the balanced equation.
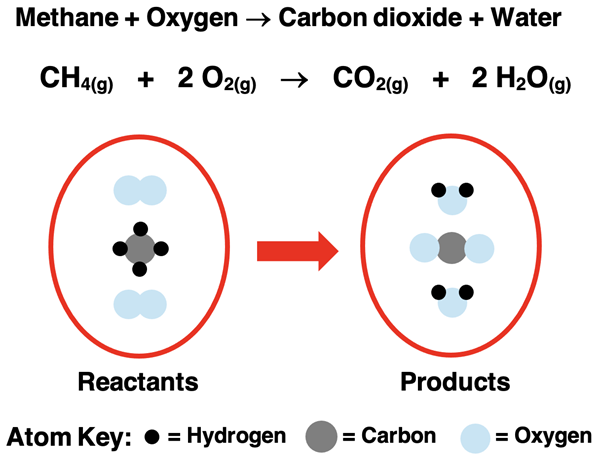
Example symbolic equation: \(\mathrm{2H_2(g) + O_2(g) \rightarrow 2H_2O(g)}\)
The corresponding particulate model would show:
- 2 diatomic hydrogen molecules (\(\mathrm{H_2}\)) reacting with 1 diatomic oxygen molecule (\(\mathrm{O_2}\)).
- Products: 2 water molecules (\(\mathrm{H_2O}\)), each consisting of two hydrogen atoms bonded to one oxygen atom.
- No atoms are gained or lost — only rearranged.
4. Particulate Representation of Physical Processes: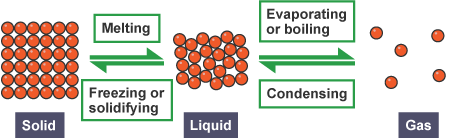
Example — Phase Change: \(\mathrm{H_2O(s) \rightarrow H_2O(l)}\) In the particulate model:
- Before: Water molecules are arranged in a fixed, ordered lattice (ice).
- After: Water molecules move more freely while remaining in close contact (liquid).
- Molecular composition remains \(\mathrm{H_2O}\); only the arrangement and motion of particles change.
5. Particulate Representation of Chemical Processes:
Example — Precipitation Reaction:
\(\mathrm{AgNO_3(aq) + NaCl(aq) \rightarrow AgCl(s) + NaNO_3(aq)}\)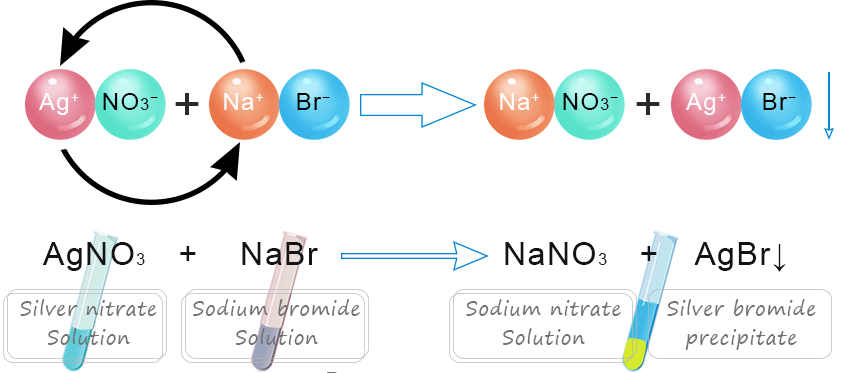
Before reaction:
- \(\mathrm{Ag^+(aq)}\) and \(\mathrm{NO_3^-(aq)}\) ions are dissociated in water.
- \(\mathrm{Na^+(aq)}\) and \(\mathrm{Cl^-(aq)}\) ions are also dissociated.
During reaction:
- \(\mathrm{Ag^+(aq)}\) and \(\mathrm{Cl^-(aq)}\) combine to form insoluble \(\mathrm{AgCl(s)}\) — visible as a solid precipitate.
- \(\mathrm{Na^+}\) and \(\mathrm{NO_3^-}\) remain free in solution as spectator ions.
Particulate-level representations are a visual translation of balanced equations. They help connect macroscopic observations (e.g., color change, gas formation) with the microscopic rearrangements of atoms and ions that obey the laws of conservation of mass and charge.
Example:
Represent the reaction of hydrogen gas with chlorine gas to form hydrogen chloride using both symbolic and particulate models.
▶️ Answer / Explanation
Step 1: Write the balanced equation: \(\mathrm{H_2(g) + Cl_2(g) \rightarrow 2HCl(g)}\)
Step 2: Symbolic interpretation:
- 1 molecule of hydrogen reacts with 1 molecule of chlorine.
- Produces 2 molecules of hydrogen chloride gas.
Step 3: Particulate representation:
- Before: Two diatomic molecules — one \(\mathrm{H_2}\) and one \(\mathrm{Cl_2}\).
- After: Two heteronuclear molecules — each with one H atom bonded to one Cl atom.
Step 4: Conservation:
- Hydrogen atoms: 2 on each side
- Chlorine atoms: 2 on each side
- Mass and charge conserved
Final Explanation: The particulate model clearly illustrates that atoms are rearranged but conserved during the reaction, consistent with the balanced chemical equation.
