AP Chemistry 4.4 Physical and Chemical Changes Study Notes - New Syllabus Effective fall 2024
AP Chemistry 4.4 Physical and Chemical Changes Study Notes- New syllabus
AP Chemistry 4.4 Physical and Chemical Changes Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship between macroscopic characteristics and bond interactions for:
i. Chemical processes.
ii. Physical processes.
Key Concepts:
- Classification of Physical and Chemical Processes
- Borderline Cases Between Physical and Chemical Processes
Classification of Physical and Chemical Processes
All processes in chemistry can be broadly classified as either physical or chemical based on whether they involve changes in the identity of the substance. The key distinction lies in whether chemical bonds are broken or formed within molecules, or if only intermolecular interactions are altered.
1. Chemical Processes: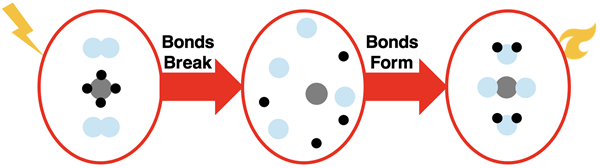
- Involve the breaking and/or formation of chemical bonds within or between molecules.
- Lead to the formation of new substances with different compositions and properties.
- In these reactions, atoms are rearranged, but the total number of each type of atom remains constant.
Examples of Chemical Processes:
![]()
- Combustion of hydrocarbons: \(\mathrm{CH_4(g) + 2O_2(g) \rightarrow CO_2(g) + 2H_2O(g)}\)
- Electrolysis of water: \(\mathrm{2H_2O(l) \rightarrow 2H_2(g) + O_2(g)}\)
- Rusting of iron: \(\mathrm{4Fe(s) + 3O_2(g) \rightarrow 2Fe_2O_3(s)}\)
Each of these examples involves bond breaking and bond formation, producing new substances with new chemical properties.
2. Physical Processes: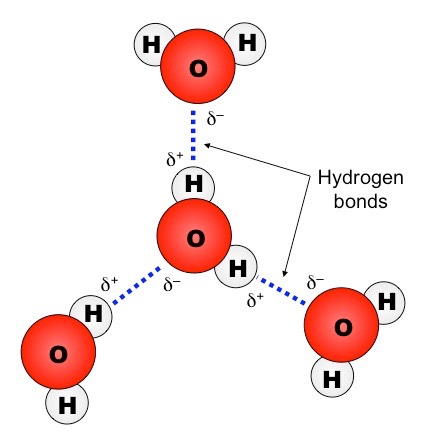
- Involve changes in intermolecular interactions, not in the actual chemical bonds between atoms.
- The chemical composition of the substance remains unchanged — only the physical form, phase, or arrangement of molecules changes.
- These processes are often reversible (e.g., melting, boiling, condensation).
Examples of Physical Processes:
![]()
- Phase changes: \(\mathrm{H_2O(s) \leftrightharpoons H_2O(l) \leftrightharpoons H_2O(g)}\)
- Dissolution of sugar in water (no bond breaking within molecules): \(\mathrm{C_{12}H_{22}O_{11}(s) \rightarrow C_{12}H_{22}O_{11}(aq)}\)
- Expansion or compression of gases.
These processes involve changes in particle arrangement or spacing, not in molecular structure.
3. Molecular Perspective:
| Type of Process | Bonds Affected | Change in Composition? | Example |
|---|---|---|---|
| Chemical | Intramolecular (within molecules) | Yes | Combustion of methane |
| Physical | Intermolecular (between molecules) | No | Melting of ice |
4. Energy Considerations:
- Both physical and chemical changes involve energy transfer (as heat or work), but the scale differs.
- Chemical processes involve much larger energy changes because bond breaking and formation occur (in the range of hundreds of kJ/mol).
- Physical changes generally involve smaller energy changes associated with intermolecular forces (e.g., hydrogen bonding, London dispersion).
Processes involving bond breaking and formation within molecules are classified as chemical, while those involving only intermolecular interactions (without altering molecular identity) are classified as physical.
Example:
Classify each process as physical or chemical and justify your answer:
(a) Melting of ice
(b) Burning of natural gas
(c) Condensation of steam
(d) Decomposition of hydrogen peroxide
▶️ Answer / Explanation
(a) Melting of ice: Physical — involves breaking hydrogen bonds (intermolecular) but no change in molecular identity (\(\mathrm{H_2O}\) remains the same).
(b) Burning of natural gas: Chemical — methane reacts with oxygen to form \(\mathrm{CO_2}\) and \(\mathrm{H_2O}\), new substances form.
(c) Condensation of steam: Physical — involves formation of intermolecular attractions; \(\mathrm{H_2O}\) remains unchanged.
(d) Decomposition of hydrogen peroxide: Chemical — new substances (\(\mathrm{H_2O}\) and \(\mathrm{O_2}\)) form via bond breaking and formation.
Borderline Cases Between Physical and Chemical Processes
Although physical and chemical processes are generally distinct, some processes such as the dissolution of ionic compounds in water—can exhibit characteristics of both. In these cases, chemical bonds are broken and new intermolecular forces are formed, making the classification as purely “physical” or “chemical” ambiguous.
1. The Dissolution Process:
When an ionic solid (e.g., salt) dissolves in water, the process involves two simultaneous steps:
- Breaking ionic bonds in the solid lattice (requires energy — endothermic).
- Forming ion–dipole interactions between the ions and the polar water molecules (releases energy — exothermic).
Overall, the process can be represented as:
\(\mathrm{NaCl(s) \rightarrow Na^+(aq) + Cl^-(aq)}\)
Here, the solid lattice of sodium chloride dissociates into free hydrated ions in aqueous solution.
2. Why It Can Be Considered a Physical Process:
- No new chemical substances are formed — sodium and chloride ions were already present in the compound.
- The process can be reversed by evaporation of the solvent (water), which re-forms the solid salt.
- The overall chemical composition of the solute and solvent remains unchanged.
- Thus, dissolution is typically classified as a physical process.
Example: When table salt (\(\mathrm{NaCl}\)) dissolves in water, the ions separate but no chemical reaction occurs between sodium and chlorine atoms.
3. Why It Can Also Be Considered a Chemical Process:
- The breaking of ionic bonds and formation of ion–dipole interactions with water molecules involve changes in bonding forces at the atomic level.
- The process involves a measurable enthalpy change (heat absorbed or released).
- The ions interact specifically with solvent molecules, forming new ion–solvent complexes (e.g., \(\mathrm{[Na(H_2O)_6]^+}\)).
- These new interactions can alter the system’s structure and energy — features typically associated with chemical processes.
Hence, from an energetic and molecular perspective, dissolution involves both bond breaking and bond formation.
4. Energy Considerations in Dissolution:
The overall energy change (\(\mathrm{\Delta H_{solution}}\)) depends on three component steps:
- \(\mathrm{\Delta H_1}\): Energy required to break ionic bonds in the solute (endothermic)
- \(\mathrm{\Delta H_2}\): Energy required to separate water molecules to make room for ions (endothermic)
- \(\mathrm{\Delta H_3}\): Energy released during ion–dipole attraction formation (exothermic)
\(\mathrm{\Delta H_{solution} = \Delta H_1 + \Delta H_2 + \Delta H_3}\)
If \(\mathrm{\Delta H_{solution}}\) is negative → dissolution is exothermic (e.g., \(\mathrm{CaCl_2}\) in water). If \(\mathrm{\Delta H_{solution}}\) is positive → dissolution is endothermic (e.g., \(\mathrm{NH_4NO_3}\) in water).
5. Molecular Representation of Dissolution:
- In the solid, ions are arranged in a rigid, repeating 3D lattice held by strong electrostatic attractions.
- In aqueous solution, ions are surrounded by water molecules oriented according to polarity:
- Oxygen (δ–) faces cations (\(\mathrm{Na^+}\)).
- Hydrogen (δ+) faces anions (\(\mathrm{Cl^-}\)).
This formation of hydration shells stabilizes the ions in solution.
| Aspect | Physical Process View | Chemical Process View |
|---|---|---|
| Type of Bonds Affected | Only intermolecular forces between solute and solvent | Break ionic bonds and form ion–dipole interactions |
| Change in Composition | No new chemical species | Ions interact with solvent to form hydrated complexes |
| Reversibility | Reversible by evaporation | Not fully reversible in some cases (e.g., hydration shells remain) |
The dissolution of an ionic solid in water is a borderline process that can be interpreted as either physical or chemical. It involves both breaking ionic bonds (a chemical aspect) and forming ion–dipole attractions (a physical interaction), highlighting that the distinction between physical and chemical changes is not always absolute.
Example:
Explain whether the dissolution of \(\mathrm{NH_4NO_3(s)}\) in water is a physical or chemical process.
▶️ Answer / Explanation
Step 1: Write the process: \(\mathrm{NH_4NO_3(s) \rightarrow NH_4^+(aq) + NO_3^-(aq)}\)
Step 2: Analyze bonding changes:
- Ionic bonds between \(\mathrm{NH_4^+}\) and \(\mathrm{NO_3^-}\) are broken (requires energy).
- Ion–dipole interactions form between water and the ions (releases energy).
Step 3: Classify the process:
- No new chemical species — suggests physical process.
- However, bond breaking and energy change — suggests chemical aspects.
Step 4: Observation:
- The solution becomes cold — endothermic process.
Final Answer: The dissolution of \(\mathrm{NH_4NO_3}\) is primarily a physical process involving ionic bond breaking and ion–dipole formation, but it exhibits chemical-like energy changes, making it a borderline case.
