AP Chemistry 4.5 Stoichiometry Study Notes - New Syllabus Effective fall 2024
AP Chemistry 4.5 Stoichiometry Study Notes- New syllabus
AP Chemistry 4.5 Stoichiometry Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain changes in the amounts of reactants and products based on the balanced reaction equation for a chemical process.
Key Concepts:
- Conservation of Atoms and Quantitative Relationships in Chemical Reactions
- Stoichiometric Coefficients and Proportionality in Chemical Reactions
- Combining Stoichiometric Calculations with Gas Laws and Molarity
Conservation of Atoms and Quantitative Relationships in Chemical Reactions
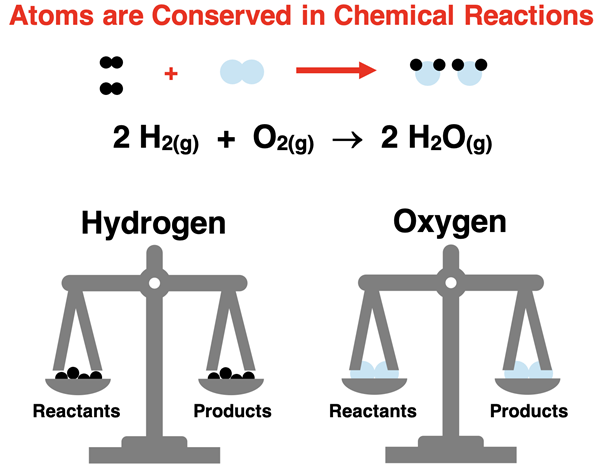
Because atoms are conserved during a chemical reaction, the amounts of reactants and products are related in fixed, predictable ratios. This principle allows chemists to calculate the amount of products formed from given reactants or vice versa based on the law of conservation of mass and the stoichiometric coefficients in a balanced chemical equation.
1. Conservation of Atoms:
- Atoms are neither created nor destroyed in chemical reactions; they are simply rearranged into new combinations.
- Therefore, the total number of each type of atom must be the same on both sides of a balanced chemical equation.
- This forms the basis for quantitative chemical calculations (stoichiometry).
Example of conservation: \(\mathrm{2H_2(g) + O_2(g) \rightarrow 2H_2O(l)}\)
- Hydrogen atoms: 4 on both sides.
- Oxygen atoms: 2 on both sides.
- Atoms are conserved.
2. Quantitative Meaning of Conservation:
Since atoms are conserved, their masses and mole ratios are also conserved across the reaction. This means we can relate the quantities of substances using the coefficients in the balanced chemical equation.
For the above example:
\(\mathrm{2H_2 + O_2 \rightarrow 2H_2O}\)
Interpretation:
- 2 moles of hydrogen react with 1 mole of oxygen to form 2 moles of water.
- This ratio holds true at all scales — from individual molecules to laboratory moles.
3. Stoichiometric Ratios:
- Each coefficient in a balanced equation represents a mole ratio between reactants and products.
- These ratios are used to determine how much of one substance is needed or produced when another amount is known.
General relationship:
\(\mathrm{\dfrac{n_A}{a} = \dfrac{n_B}{b} = \dfrac{n_C}{c} = …}\)
where \(a, b, c, …\) are the stoichiometric coefficients for substances A, B, C, etc.
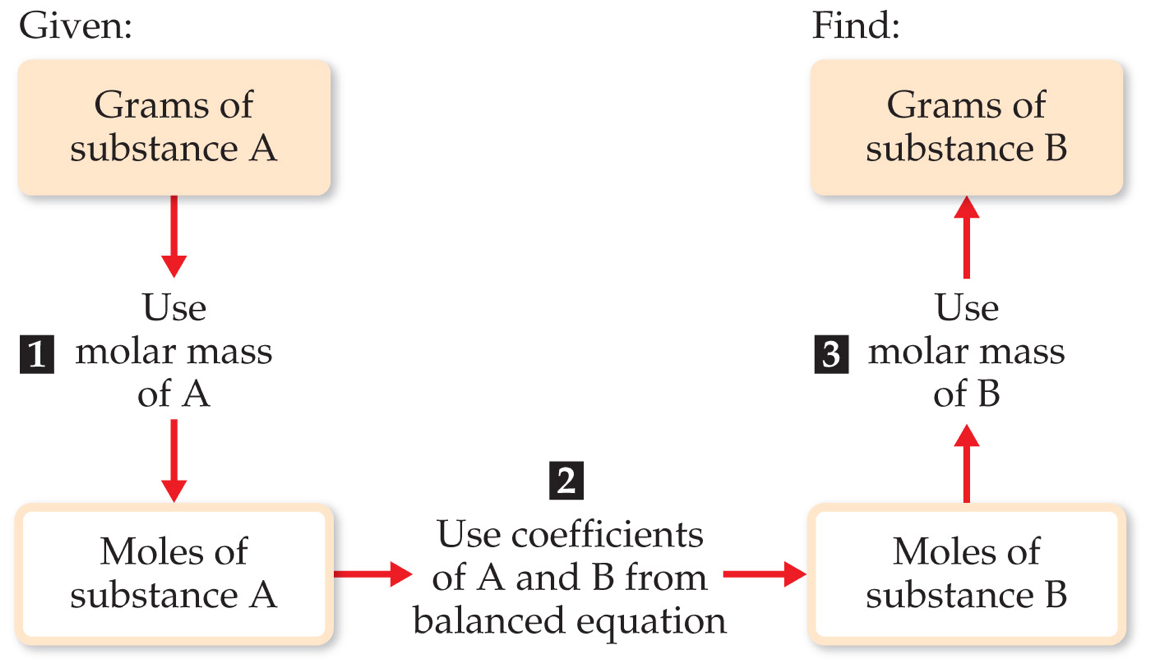
4. Steps for Quantitative Calculations Using Conservation:
- Write the balanced chemical equation.
- Convert given information (mass, volume, molarity, etc.) to moles.
- Use the mole ratio from the balanced equation to find the moles of another substance.
- Convert the result to the desired unit (mass, volume, etc.).
Because atoms and therefore mass are conserved, the amounts of reactants and products in a reaction can be calculated precisely using mole ratios derived from a balanced chemical equation.
Example:
Hydrogen gas reacts with nitrogen gas to produce ammonia according to the balanced equation:
\(\mathrm{N_2(g) + 3H_2(g) \rightarrow 2NH_3(g)}\)
Calculate the number of moles of ammonia that can be formed from 4.5 moles of hydrogen gas, assuming excess nitrogen.
▶️ Answer / Explanation
Step 1: Identify the mole ratio from the balanced equation.
From the equation: \(\mathrm{3H_2 \rightarrow 2NH_3}\)
So, the ratio is: \(\mathrm{\dfrac{2\,mol\,NH_3}{3\,mol\,H_2}}\)
Step 2: Use stoichiometry to find moles of ammonia.
\(\mathrm{4.5\,mol\,H_2 \times \dfrac{2\,mol\,NH_3}{3\,mol\,H_2} = 3.0\,mol\,NH_3}\)
Step 3: Interpret the result.
From 4.5 moles of hydrogen gas, 3.0 moles of ammonia can form if nitrogen is in excess.
Final Answer: \(\mathrm{3.0\ mol\ NH_3}\)
Stoichiometric Coefficients and Proportionality in Chemical Reactions
The coefficients in a balanced chemical equation express the proportional relationships among the amounts of reactants and products. These coefficients represent the mole ratios that govern how substances combine and react according to the law of conservation of mass.
1. Meaning of Stoichiometric Coefficients:
- Each coefficient specifies the relative number of particles (atoms, molecules, or formula units) participating in the reaction.
- At the macroscopic level, coefficients represent the relative number of moles of each species involved.
- The ratios between coefficients are constant for a given chemical reaction, regardless of scale.
Example: \(\mathrm{2H_2(g) + O_2(g) \rightarrow 2H_2O(l)}\)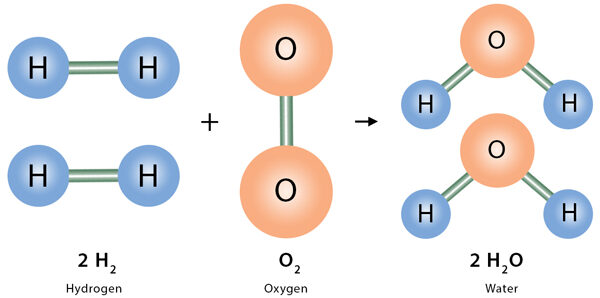
- 2 molecules (or moles) of hydrogen react with 1 molecule (or mole) of oxygen.
- They produce 2 molecules (or moles) of water.
- Ratio: \(\mathrm{H_2 : O_2 : H_2O = 2 : 1 : 2}\)
2. Quantitative Relationships from Coefficients:
The stoichiometric coefficients can be used to determine relationships between the amounts of reactants and products in any reaction. These relationships include:
- Mole–Mole relationship: From balanced equation: \(\mathrm{aA + bB \rightarrow cC + dD}\)
\(\mathrm{\dfrac{n_A}{a} = \dfrac{n_B}{b} = \dfrac{n_C}{c} = \dfrac{n_D}{d}}\)
- Mass–Mass relationship: Using molar mass (\(\mathrm{g/mol}\)), we can convert between mass and moles.
- Mass–Mole relationship: Given one substance’s mass, convert to moles, use mole ratio, then convert back to mass for another substance.
3. Using Coefficients for Quantitative Calculations: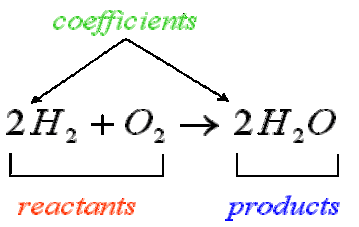
The steps are as follows:
- Write and balance the chemical equation.
- Convert given quantity (mass, volume, or molarity) to moles.
- Use the stoichiometric ratio from coefficients to find the moles of another substance.
- Convert moles back to desired units (mass, volume, or particles).
Equation for mole relationships:
\(\mathrm{moles\ of\ A \times \dfrac{coefficient\ of\ B}{coefficient\ of\ A} = moles\ of\ B}\)
4. Conceptual Application of Coefficients:
- The coefficients define how far a reaction can proceed and what the theoretical yield will be.
- They also determine which reactant limits the reaction (limiting reagent concept).
- Ratios are consistent across any scale microscopic, laboratory, or industrial.
5. Example Relationships:
| Type of Relationship | Equation or Concept | Example |
|---|---|---|
| Mole Ratio | \(\mathrm{aA + bB \rightarrow cC + dD}\) | \(\mathrm{2H_2 + O_2 \rightarrow 2H_2O}\) |
| Mole–Mass | Use molar mass to relate mass to moles | 2 mol \(\mathrm{H_2}\) = 4.04 g |
| Mass–Mass | Convert given mass → moles → moles → mass | 36 g water from 4 g hydrogen |
The stoichiometric coefficients of a balanced equation reveal the fixed ratios among substances in a reaction. They allow quantitative predictions of how much product will form from given reactants and ensure consistency with the conservation of mass.
Example:
Given the reaction: \(\mathrm{2Al(s) + 3Cl_2(g) \rightarrow 2AlCl_3(s)}\)
How many moles of aluminum chloride are produced from 5.00 moles of chlorine gas, assuming excess aluminum?
▶️ Answer / Explanation
Step 1: Identify mole ratio.
From balanced equation: \(\mathrm{3Cl_2 \rightarrow 2AlCl_3}\)
Ratio: \(\mathrm{\dfrac{2\,mol\,AlCl_3}{3\,mol\,Cl_2}}\)
Step 2: Use stoichiometric ratio.
\(\mathrm{5.00\,mol\,Cl_2 \times \dfrac{2\,mol\,AlCl_3}{3\,mol\,Cl_2} = 3.33\,mol\,AlCl_3}\)
Step 3: Interpret result.
From 5.00 mol of chlorine gas, 3.33 mol of aluminum chloride can form.
Final Answer: \(\mathrm{3.33\ mol\ AlCl_3}\)
Combining Stoichiometric Calculations with Gas Laws and Molarity
Stoichiometric relationships derived from balanced chemical equations can be extended to gaseous and aqueous systems by incorporating the Ideal Gas Law and molarity relationships. This integration allows chemists to perform quantitative studies of gases and solutions, connecting macroscopic measurements (pressure, volume, temperature, concentration) with mole-based stoichiometry.
1. Stoichiometry and the Ideal Gas Law: 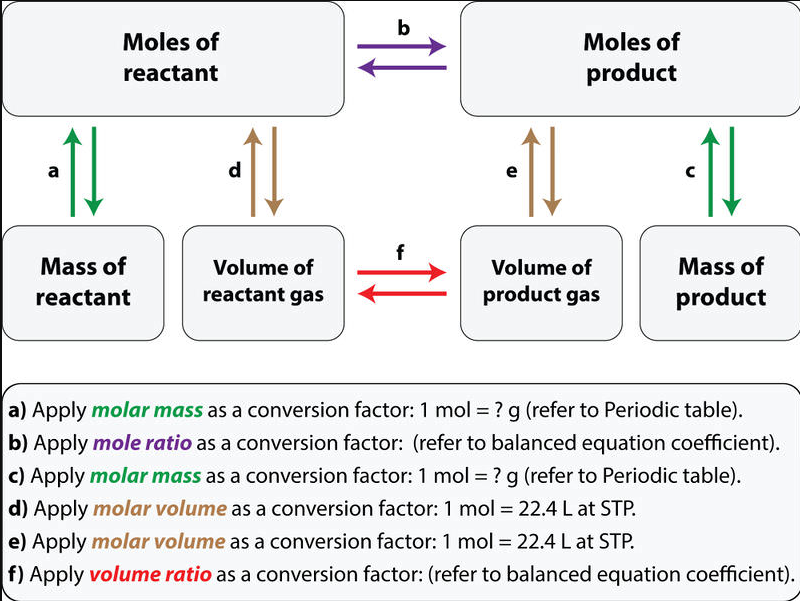
The Ideal Gas Law relates the measurable properties of a gas to its amount in moles:
\(\mathrm{PV = nRT}\)
- \(\mathrm{P}\) = pressure (atm)
- \(\mathrm{V}\) = volume (L)
- \(\mathrm{n}\) = moles of gas
- \(\mathrm{R}\) = ideal gas constant (\(0.0821\ \mathrm{L·atm·mol^{-1}·K^{-1}}\))
- \(\mathrm{T}\) = temperature (K)
Application: In reactions involving gases, the volume of a gas can be related directly to its moles, and therefore to the stoichiometric coefficients in the balanced equation.
Example : If 1 mol of \(\mathrm{N_2}\) reacts with 3 mol of \(\mathrm{H_2}\) to form 2 mol of \(\mathrm{NH_3}\), then at the same temperature and pressure, 1 L of \(\mathrm{N_2}\) reacts with 3 L of \(\mathrm{H_2}\) to produce 2 L of \(\mathrm{NH_3}\).
2. Stoichiometry and Molarity (Solutions):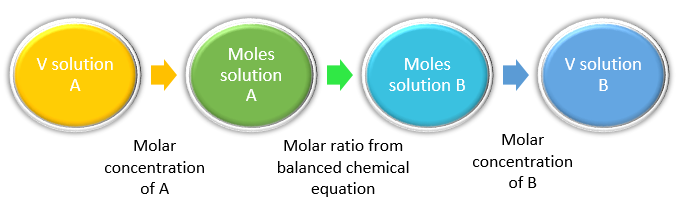
In aqueous reactions, quantities are often given in terms of molarity (M) — moles of solute per liter of solution.
\(\mathrm{M = \dfrac{n_{solute}}{V_{solution}}}\)
Thus, the number of moles can be calculated as:
\(\mathrm{n = M \times V}\)
Application: This allows stoichiometric calculations to link solution concentrations to reaction quantities, for example in titrations or precipitation reactions.
Example : \(\mathrm{M_1V_1 = M_2V_2}\) (for reactions involving 1:1 stoichiometry between acid and base).
3. Integrated Stoichiometric Calculations:
In many problems, both gaseous and aqueous species are involved. The following relationships can be combined:
- Ideal Gas Law → to find moles of gas

- Molarity → to find moles of solute
- Stoichiometry → to relate moles of reactants and products
Flowchart for combined calculations:
\(\mathrm{(P, V, T) \xrightarrow{PV = nRT} n_{gas} \xrightarrow{stoichiometry} n_{product} \xrightarrow{M = \frac{n}{V}} M_{solution}}\)
4. Common Integrated Problem Types:
- Finding gas volume produced or consumed at given temperature and pressure.
- Determining molarity or concentration change after a reaction.
- Calculating limiting reagent when one reactant is a gas and another is in solution.
5. Key Quantitative Relationships:
| Quantity | Relationship | Used To Find |
|---|---|---|
| Gas Moles | \(\mathrm{n = \dfrac{PV}{RT}}\) | Moles from pressure, volume, temperature |
| Solution Moles | \(\mathrm{n = M \times V}\) | Moles from concentration and volume |
| Stoichiometric Relation | \(\mathrm{n_A \times \dfrac{coeff_B}{coeff_A} = n_B}\) | Relate reactant and product moles |
Example:
Hydrochloric acid reacts with sodium carbonate according to:
\(\mathrm{Na_2CO_3(aq) + 2HCl(aq) \rightarrow 2NaCl(aq) + H_2O(l) + CO_2(g)}\)
Calculate the volume of \(\mathrm{CO_2}\) gas produced at \(298\ \mathrm{K}\) and \(1.00\ \mathrm{atm}\) when \(100.0\ \mathrm{mL}\) of \(0.200\ \mathrm{M\ HCl}\) reacts with excess sodium carbonate.
▶️ Answer / Explanation
Step 1: Find moles of \(\mathrm{HCl}\).
\(\mathrm{n_{HCl} = M \times V = 0.200\ mol/L \times 0.100\ L = 0.0200\ mol}\)
Step 2: Use stoichiometry to find moles of \(\mathrm{CO_2}\).
From the balanced equation: \(\mathrm{2HCl \rightarrow 1CO_2}\)
\(\mathrm{n_{CO_2} = 0.0200\ mol\ HCl \times \dfrac{1\ mol\ CO_2}{2\ mol\ HCl} = 0.0100\ mol\ CO_2}\)
Step 3: Apply the Ideal Gas Law to find the volume of \(\mathrm{CO_2}\).
\(\mathrm{V = \dfrac{nRT}{P} = \dfrac{(0.0100)(0.0821)(298)}{1.00} = 0.245\ L}\)
Step 4: Convert to milliliters.
\(\mathrm{0.245\ L = 245\ mL}\)
Final Answer: \(\mathrm{245\ mL\ of\ CO_2(g)}\) is produced under the given conditions.