AP Chemistry 4.7 Types of Chemical Reactions Study Notes - New Syllabus Effective fall 2024
AP Chemistry 4.7 Types of Chemical Reactions Study Notes- New syllabus
AP Chemistry 4.7 Types of Chemical Reactions Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Identify a reaction as acid-base, oxidation-reduction, or precipitation.
Key Concepts:
- Acid–Base Reactions and Proton Transfer
- Oxidation–Reduction (Redox) Reactions and Combustion
- Electron Transfer in Redox Reactions
- Oxidation Numbers and Identifying Redox Changes
- Precipitation Reactions and Solubility Rules
Acid–Base Reactions and Proton Transfer
Acid–base reactions involve the transfer of one or more protons (\( \mathrm{H^+} \)) between chemical species. These reactions are central to understanding the behavior of solutions, equilibrium, and reaction mechanisms in aqueous systems.
Key Concepts: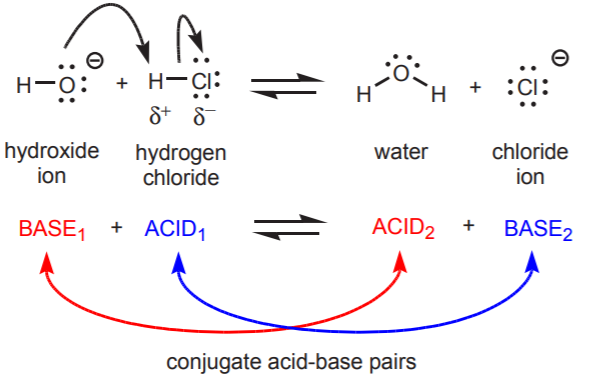
- An acid is a proton (\( \mathrm{H^+} \)) donor.
- A base is a proton (\( \mathrm{H^+} \)) acceptor.
- When an acid donates a proton, it becomes its conjugate base.
- When a base accepts a proton, it becomes its conjugate acid.
General Representation:
\( \mathrm{HA + B \rightleftharpoons A^- + HB^+} \)
- \( \mathrm{HA} \): acid (proton donor)
- \( \mathrm{B} \): base (proton acceptor)
- \( \mathrm{A^-} \): conjugate base of the acid
- \( \mathrm{HB^+} \): conjugate acid of the base
In every acid–base reaction, there are two conjugate acid–base pairs that differ by one proton. Proton transfer is the fundamental process that defines the reaction.
Examples of Conjugate Acid–Base Pairs:
| Acid | Conjugate Base | Base | Conjugate Acid |
|---|---|---|---|
| \( \mathrm{HCl} \) | \( \mathrm{Cl^-} \) | \( \mathrm{H_2O} \) | \( \mathrm{H_3O^+} \) |
| \( \mathrm{NH_4^+} \) | \( \mathrm{NH_3} \) | \( \mathrm{H_2O} \) | \( \mathrm{OH^-} \) |
Example
Identifying Proton Transfer in an Acid–Base Reaction , Consider the reaction between ammonia and water:
\( \mathrm{NH_3 + H_2O \rightleftharpoons NH_4^+ + OH^-} \)
▶️ Answer / Explanation
Step 1: Identify the acid and base on the left side.
- \( \mathrm{NH_3} \) accepts a proton → it acts as a base.
- \( \mathrm{H_2O} \) donates a proton → it acts as an acid.
Step 2: Identify the conjugate pairs formed on the right side.
- \( \mathrm{NH_4^+} \) is the conjugate acid of \( \mathrm{NH_3} \).
- \( \mathrm{OH^-} \) is the conjugate base of \( \mathrm{H_2O} \).
Step 3: Describe the proton transfer.
A proton is transferred from water to ammonia, forming \( \mathrm{NH_4^+} \) and \( \mathrm{OH^-} \).
Result: This reaction demonstrates that acid–base behavior is governed by the transfer of one or more protons between species, forming conjugate acid–base pairs.
Oxidation–Reduction (Redox) Reactions and Combustion
Oxidation–reduction (redox) reactions involve the transfer of one or more electrons between chemical species. These reactions are identified by changes in the oxidation numbers of the elements involved.
Key Concepts:
- Oxidation is the loss of electrons, resulting in an increase in oxidation number.

- Reduction is the gain of electrons, resulting in a decrease in oxidation number.
- The species that donates electrons is the reducing agent (it gets oxidized).
- The species that accepts electrons is the oxidizing agent (it gets reduced).
- Redox reactions always occur in pairs: one species is oxidized while another is reduced.
General Representation:
\( \mathrm{Oxidation:\; A \rightarrow A^{n+} + ne^-} \)
\( \mathrm{Reduction:\; B^{m+} + ne^- \rightarrow B} \)
Key Idea: In a redox process, electrons are transferred from the species being oxidized to the species being reduced, changing their oxidation states and conserving overall charge and mass.
Recognizing Redox Reactions:
![]()
- Track changes in oxidation numbers for all elements involved.
- If an element’s oxidation number increases → it is oxidized.
- If an element’s oxidation number decreases → it is reduced.
Special Case — Combustion Reactions:
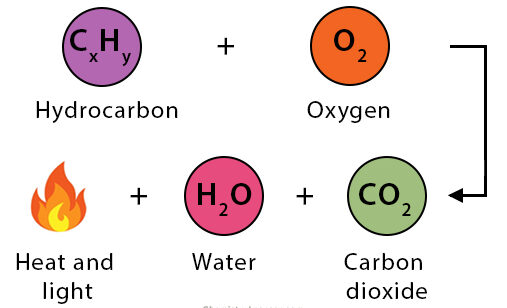
- Combustion is a subclass of redox reactions in which a substance reacts with oxygen gas (\( \mathrm{O_2} \)).
- Combustion reactions are typically highly exothermic.
- For hydrocarbons, complete combustion produces carbon dioxide (\( \mathrm{CO_2} \)) and water (\( \mathrm{H_2O} \)).
General Equation for Hydrocarbon Combustion:
\(\mathrm{C_{x}H_y + O_2 \rightarrow CO_2 + H_2O}\)
| Process | Definition | Electron Flow | Change in Oxidation Number |
|---|---|---|---|
| Oxidation | Loss of electrons | Outward (electrons released) | Increases |
| Reduction | Gain of electrons | Inward (electrons accepted) | Decreases |
Example
Identify Oxidation and Reduction , Consider the reaction:
\( \mathrm{CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O} \)
▶️ Answer / Explanation
Step 1: Assign oxidation numbers.
- \( \mathrm{C} \) in \( \mathrm{CH_4} \): –4
- \( \mathrm{C} \) in \( \mathrm{CO_2} \): +4
- \( \mathrm{O} \) in \( \mathrm{O_2} \): 0
- \( \mathrm{O} \) in \( \mathrm{H_2O} \): –2
Step 2: Determine changes in oxidation number.
- Carbon: from –4 to +4 → oxidized (loss of 8 electrons per molecule of methane).
- Oxygen: from 0 to –2 → reduced (gain of electrons).
Step 3: Identify agents.
- \( \mathrm{CH_4} \) is the reducing agent.
- \( \mathrm{O_2} \) is the oxidizing agent.
Result: This is a combustion reaction, a redox process where methane is oxidized to carbon dioxide and oxygen is reduced to water.
Electron Transfer in Redox Reactions
In a redox (oxidation–reduction) reaction, electrons are transferred from the species that is oxidized to the species that is reduced. This transfer of electrons links the oxidation and reduction half-reactions, conserving both charge and mass.
Key Concepts: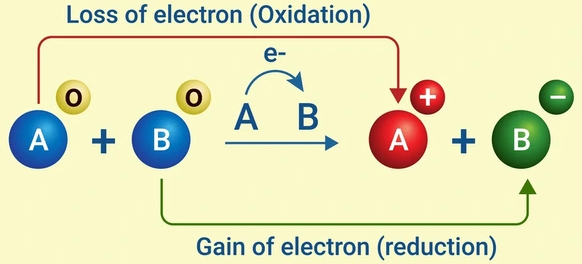
- The oxidized species loses electrons and acts as the reducing agent.
- The reduced species gains electrons and acts as the oxidizing agent.
- Electrons flow from the reducing agent to the oxidizing agent.
- Each redox reaction can be separated into two half-reactions — one for oxidation and one for reduction — which together maintain charge balance.
General Representation:
Oxidation half: \( \mathrm{A \rightarrow A^{n+} + ne^-} \)
Reduction half: \( \mathrm{B^{m+} + ne^- \rightarrow B} \)
Overall redox reaction:
\( \mathrm{A + B^{m+} \rightarrow A^{n+} + B} \)
- \( \mathrm{A} \): species that is oxidized (loses electrons)
- \( \mathrm{B^{m+}} \): species that is reduced (gains electrons)
- Electrons (\( \mathrm{e^-} \)) are not shown in the overall balanced equation because they are transferred internally.
Redox reactions always involve a direct transfer of electrons — oxidation and reduction occur simultaneously. The substance oxidized loses electrons, which are gained by the substance reduced.
| Process | Species Role | Electron Behavior | Agent Type |
|---|---|---|---|
| Oxidation | Electron donor | Loses electrons | Reducing agent |
| Reduction | Electron acceptor | Gains electrons | Oxidizing agent |
Example
Describe Electron Transfer in a Redox Reaction Consider the reaction between zinc metal and copper(II) ions:
\( \mathrm{Zn(s) + Cu^{2+}(aq) \rightarrow Zn^{2+}(aq) + Cu(s)} \)
▶️ Answer / Explanation
Step 1: Write the oxidation and reduction half-reactions.
- Oxidation (loss of electrons): \( \mathrm{Zn \rightarrow Zn^{2+} + 2e^-} \)
- Reduction (gain of electrons): \( \mathrm{Cu^{2+} + 2e^- \rightarrow Cu} \)
Step 2: Identify electron flow.
Electrons move from zinc (the reducing agent) to copper ions (the oxidizing agent).
Step 3: Determine oxidation and reduction roles.
- \( \mathrm{Zn} \) is oxidized → reducing agent.
- \( \mathrm{Cu^{2+}} \) is reduced → oxidizing agent.
Result: Electrons are transferred from zinc to copper(II) ions, producing \( \mathrm{Zn^{2+}} \) and metallic copper. This direct electron transfer defines the redox process.
Oxidation Numbers and Identifying Redox Changes
Oxidation numbers (also called oxidation states) are numerical values assigned to atoms in compounds or ions that represent the apparent charge each atom would have if all bonding electrons were assigned to the more electronegative element. Assigning oxidation numbers allows us to identify which species are oxidized and which are reduced in a redox reaction.
Purpose: By comparing oxidation numbers of atoms in the reactants and products, we can determine:![]()
- Which element is oxidized (oxidation number increases)
- Which element is reduced (oxidation number decreases)
Key Rules for Assigning Oxidation Numbers:
| Rule | Description | Example |
|---|---|---|
| 1 | Free (uncombined) elements have oxidation number 0 | \( \mathrm{O_2, N_2, Cl_2, Fe} \) |
| 2 | Monatomic ions have oxidation numbers equal to their charge | \( \mathrm{Na^+ = +1,\; Cl^- = -1} \) |
| 3 | Oxygen is usually –2 (except in peroxides, where it is –1) | \( \mathrm{H_2O: O = -2;\; H_2O_2: O = -1} \) |
| 4 | Hydrogen is usually +1 (except when bonded to metals, where it is –1) | \( \mathrm{HCl: H = +1;\; NaH: H = -1} \) |
| 5 | Sum of oxidation numbers in a neutral compound is 0; in a polyatomic ion, it equals the ion’s charge | \( \mathrm{H_2SO_4: 2(+1) + S + 4(-2) = 0} \) |
Comparing oxidation numbers of elements in the reactants and products is a reliable method to identify which species undergo oxidation (loss of electrons) and reduction (gain of electrons) in redox reactions.
Example
Using Oxidation Numbers to Identify Oxidized and Reduced Species , Consider the reaction:
\( \mathrm{2Na + Cl_2 \rightarrow 2NaCl} \)
▶️ Answer / Explanation
Step 1: Assign oxidation numbers to each atom.
- \( \mathrm{Na} \) (elemental form): 0
- \( \mathrm{Cl_2} \) (elemental form): 0
- In \( \mathrm{NaCl} \): \( \mathrm{Na = +1,\; Cl = -1} \)
Step 2: Determine changes in oxidation number.
- \( \mathrm{Na: 0 \rightarrow +1} \) → oxidized
- \( \mathrm{Cl: 0 \rightarrow -1} \) → reduced
Step 3: Identify oxidizing and reducing agents.
- \( \mathrm{Na} \) is the reducing agent (it donates electrons).
- \( \mathrm{Cl_2} \) is the oxidizing agent (it accepts electrons).
Result: The oxidation number method clearly shows that sodium undergoes oxidation and chlorine undergoes reduction through electron transfer.
Precipitation Reactions and Solubility Rules
Precipitation reactions occur when two aqueous ionic solutions are mixed, and ions in the solution combine to form an insoluble or sparingly soluble solid known as a precipitate. These reactions are examples of double replacement (metathesis) reactions where ions exchange partners in solution.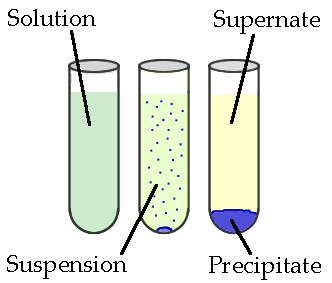
General Representation:
\( \mathrm{AB_{(aq)} + CD_{(aq)} \rightarrow AD_{(aq)} + CB_{(s)}} \)
- \( \mathrm{A^+} \) and \( \mathrm{C^+} \) are cations, while \( \mathrm{B^-} \) and \( \mathrm{D^-} \) are anions.
- The solid \( \mathrm{CB_{(s)}} \) is the precipitate formed if it is insoluble in water.
A precipitation reaction occurs only when the product of the ion exchange is insoluble or sparingly soluble in water.
Common Solubility Rules:
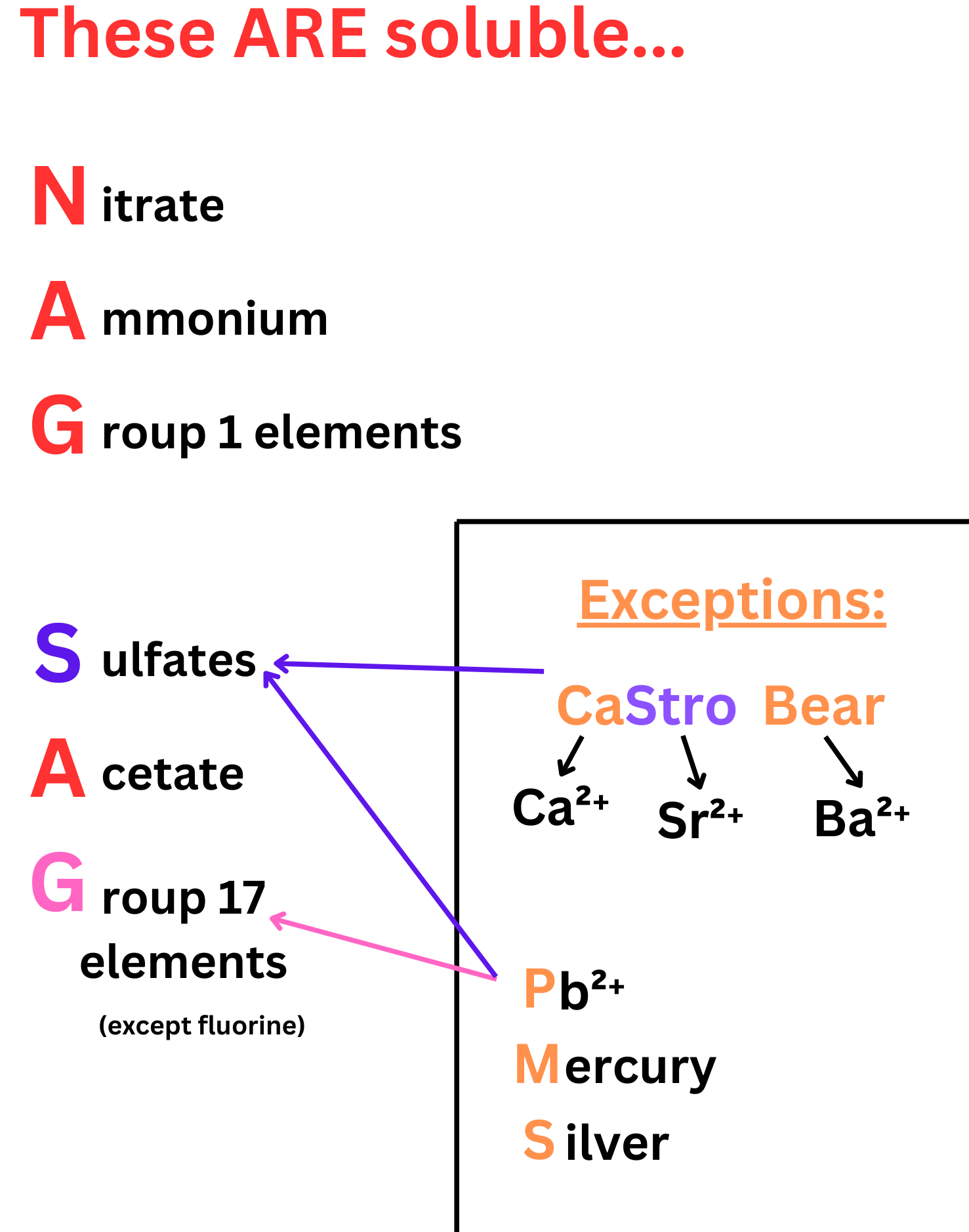
| Soluble Compounds | Insoluble Compounds (Form Precipitates) |
|---|---|
| All \( \mathrm{Na^+} \), \( \mathrm{K^+} \), and \( \mathrm{NH_4^+} \) salts | Most \( \mathrm{CO_3^{2-}} \), \( \mathrm{PO_4^{3-}} \), \( \mathrm{CrO_4^{2-}} \), and \( \mathrm{S^{2-}} \) salts (except those with \( \mathrm{Na^+} \), \( \mathrm{K^+} \), or \( \mathrm{NH_4^+} \)) |
| All \( \mathrm{NO_3^-} \) (nitrate) salts | Most \( \mathrm{OH^-} \) salts (except those with alkali metals or \( \mathrm{Ba^{2+}} \), \( \mathrm{Ca^{2+}} \), \( \mathrm{Sr^{2+}} \)) |
| Most \( \mathrm{Cl^-} \), \( \mathrm{Br^-} \), \( \mathrm{I^-} \) salts (except \( \mathrm{Ag^+} \), \( \mathrm{Pb^{2+}} \), \( \mathrm{Hg_2^{2+}} \)) | Most \( \mathrm{Ag^+} \), \( \mathrm{Pb^{2+}} \), and \( \mathrm{Hg_2^{2+}} \) halides |
| Most \( \mathrm{SO_4^{2-}} \) salts (except \( \mathrm{Ba^{2+}} \), \( \mathrm{Pb^{2+}} \), \( \mathrm{Ca^{2+}} \), \( \mathrm{Sr^{2+}} \)) | Heavy metal sulfates (\( \mathrm{PbSO_4} \), \( \mathrm{BaSO_4} \)) are insoluble |
Solubility Fact: All sodium (\( \mathrm{Na^+} \)), potassium (\( \mathrm{K^+} \)), ammonium (\( \mathrm{NH_4^+} \)), and nitrate (\( \mathrm{NO_3^-} \)) salts are soluble in water.
Net Ionic Equation Concept:
- The net ionic equation shows only the species that actually participate in the formation of the precipitate.
- Spectator ions (those that remain unchanged in solution) are omitted.
Example
When aqueous solutions of \( \mathrm{Na_2SO_4} \) and \( \mathrm{BaCl_2} \) are mixed, will a precipitate form? If yes, write the balanced molecular, ionic, and net ionic equations.
▶️ Answer / Explanation
Step 1: Write the balanced molecular equation.
\( \mathrm{Na_2SO_4(aq) + BaCl_2(aq) \rightarrow 2NaCl(aq) + BaSO_4(s)} \)
The product \( \mathrm{BaSO_4} \) is a solid precipitate (insoluble sulfate).
Step 2: Write the complete ionic equation.
\( \mathrm{2Na^+ (aq) + SO_4^{2-} (aq) + Ba^{2+} (aq) + 2Cl^- (aq) \rightarrow 2Na^+ (aq) + 2Cl^- (aq) + BaSO_4(s)} \)
Step 3: Remove spectator ions to get the net ionic equation.
\( \mathrm{Ba^{2+} (aq) + SO_4^{2-} (aq) \rightarrow BaSO_4(s)} \)
Step 4: Identify the precipitate.
\( \mathrm{BaSO_4} \) is the precipitate formed, as barium sulfate is insoluble in water.
Result: Mixing \( \mathrm{Na_2SO_4} \) and \( \mathrm{BaCl_2} \) produces a white precipitate of barium sulfate, confirming that a precipitation reaction has occurred.
