AP Chemistry 4.8 Introduction to Acid-Base Reactions Study Notes - New Syllabus Effective fall 2024
AP Chemistry 4.8 Introduction to Acid-Base Reactions Study Notes- New syllabus
AP Chemistry 4.8 Introduction to Acid-Base Reactions Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Identify species as Brønsted-Lowry acids, bases, and/or conjugate acid-base pairs, based on proton-transfer involving those species.
Key Concepts:
- Brønsted-Lowry Acids & Bases
- The Role of Water in Acid–Base Reactions
- Conjugate Acid–Base Pairs and Their Relative Strengths
Brønsted–Lowry Definition of Acids and Bases
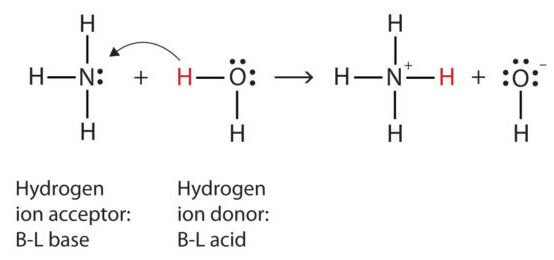
According to the Brønsted–Lowry theory, an acid is defined as a proton donor (\(\mathrm{H^+}\)) and a base is defined as a proton acceptor. This model focuses on the transfer of protons between chemical species and is applicable to both aqueous and nonaqueous systems.
1. Characteristics of Brønsted–Lowry Acids and Bases:
![]()
| Type | Definition | Example |
|---|---|---|
| Brønsted–Lowry Acid | Donates a proton (\(\mathrm{H^+}\)) to another substance. | \(\mathrm{HCl + H_2O \rightarrow H_3O^+ + Cl^-}\) |
| Brønsted–Lowry Base | Accepts a proton (\(\mathrm{H^+}\)) from another substance. | \(\mathrm{NH_3 + H_2O \rightarrow NH_4^+ + OH^-}\) |
2. Key Points of the Brønsted–Lowry Theory:
- Acid–base reactions always involve two conjugate pairs — one acid and one base on each side of the reaction.
- The acid donates a proton to the base, forming its conjugate base and the base’s conjugate acid.
- The proton transfer is the central feature of all Brønsted–Lowry reactions.
General Representation: \(\mathrm{HA + B \rightleftharpoons A^- + HB^+}\)![]()
- \(\mathrm{HA}\): Acid (proton donor)
- \(\mathrm{B}\): Base (proton acceptor)
- \(\mathrm{A^-}\): Conjugate base of the acid
- \(\mathrm{HB^+}\): Conjugate acid of the base
3. Acid–Base Conjugate Pairs:
- Each acid has a conjugate base formed by removing one proton.
- Each base has a conjugate acid formed by adding one proton.
Examples:
- \(\mathrm{HCl/Cl^-}\)
- \(\mathrm{H_2O/H_3O^+}\)
- \(\mathrm{NH_4^+/NH_3}\)
The Brønsted–Lowry model emphasizes the transfer of protons between species. An acid–base reaction cannot occur without both a proton donor and a proton acceptor present they always occur in conjugate pairs.
Example:
Identify the Brønsted–Lowry acid, base, and conjugate pairs in the reaction:
\(\mathrm{HF + H_2O \rightleftharpoons F^- + H_3O^+}\)
▶️ Answer / Explanation
- \(\mathrm{HF}\): Acid (donates a proton)
- \(\mathrm{H_2O}\): Base (accepts a proton)
- \(\mathrm{F^-}\): Conjugate base of \(\mathrm{HF}\)
- \(\mathrm{H_3O^+}\): Conjugate acid of \(\mathrm{H_2O}\)
Final Note: The proton transfer from \(\mathrm{HF}\) to \(\mathrm{H_2O}\) defines this as a Brønsted–Lowry acid–base reaction.
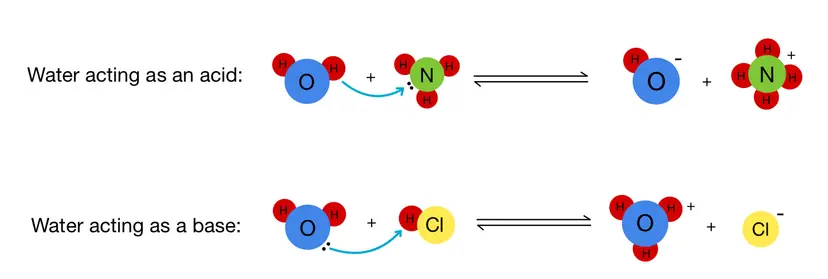
The Role of Water in Acid–Base Reactions
In aqueous solutions, water (\(\mathrm{H_2O}\)) plays a central role in many acid–base reactions because its molecular structure allows it to both donate and accept protons. This dual behavior makes water an amphoteric substance it can act as either a Brønsted–Lowry acid or a Brønsted–Lowry base depending on the reacting species.
1. Amphoteric Nature of Water: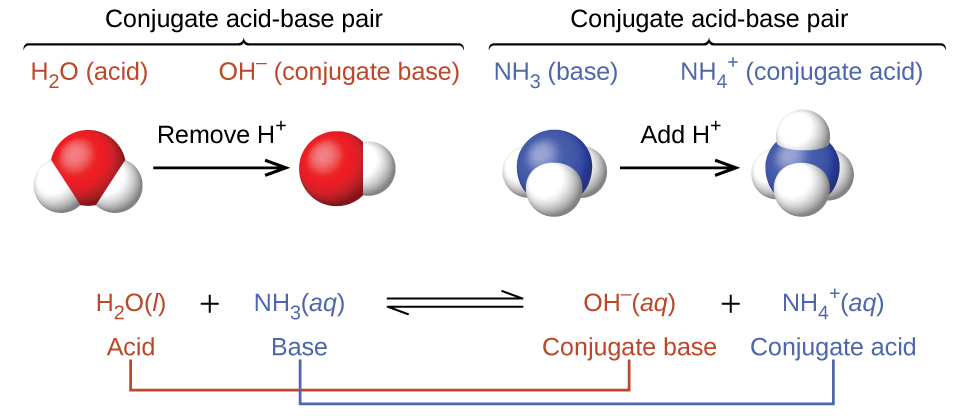
- Water can act as a proton donor (acid) or proton acceptor (base).
- This property is due to the presence of both:
- a lone pair of electrons on oxygen (allowing proton acceptance)
- a polar O–H bond (allowing proton donation)
- Therefore, water can participate in both acidic and basic reactions.
2. Water Acting as a Base:
When an acid is dissolved in water, water acts as the proton acceptor (base).
Example: \(\mathrm{HCl + H_2O \rightarrow H_3O^+ + Cl^-}\)
- \(\mathrm{HCl}\): Acid (proton donor)
- \(\mathrm{H_2O}\): Base (proton acceptor)
- \(\mathrm{H_3O^+}\): Conjugate acid (hydronium ion)

- \(\mathrm{Cl^-}\): Conjugate base
3. Water Acting as an Acid:
When a base is dissolved in water, water acts as the proton donor (acid).
Example: \(\mathrm{NH_3 + H_2O \rightleftharpoons NH_4^+ + OH^-}\)
- \(\mathrm{NH_3}\): Base (proton acceptor)
- \(\mathrm{H_2O}\): Acid (proton donor)
- \(\mathrm{NH_4^+}\): Conjugate acid
- \(\mathrm{OH^-}\): Conjugate base
4. Autoionization (Self-Ionization) of Water:
Even pure water undergoes a very small degree of ionization in which two water molecules react with each other: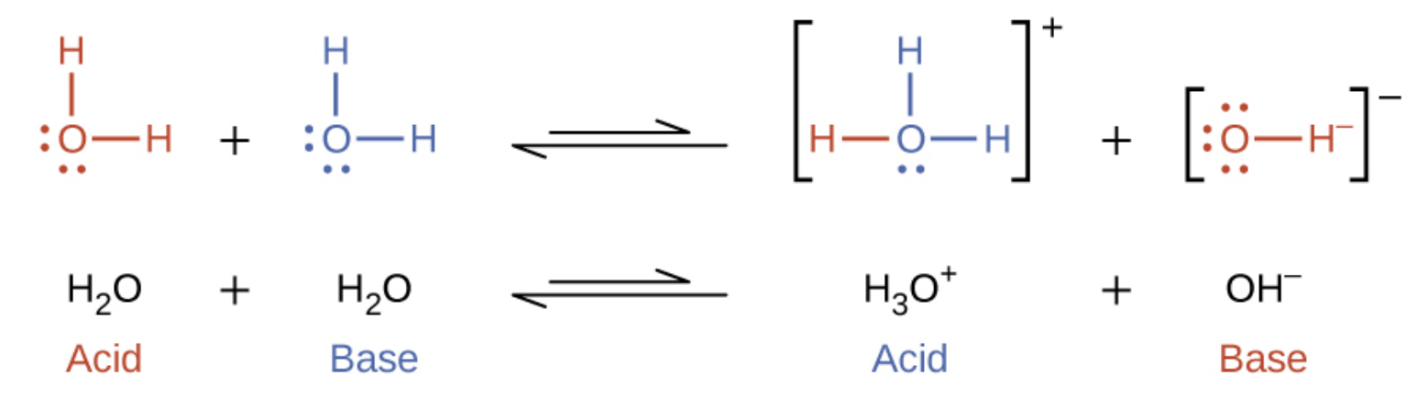
\(\mathrm{2H_2O(l) \rightleftharpoons H_3O^+(aq) + OH^-(aq)}\)
- This process establishes the ion-product constant for water (\(\mathrm{K_w}\)).
- At \(25^\circ \mathrm{C}\): \(\mathrm{K_w = [H_3O^+][OH^-] = 1.0 \times 10^{-14}}\).
- In pure water: \(\mathrm{[H_3O^+] = [OH^-] = 1.0 \times 10^{-7}\ M}\).
Significance: The autoionization of water defines the neutral point (pH 7) and provides the foundation for the pH scale.
5. Amphoteric Substances Beyond Water:
Other molecules and ions that can act as both acids and bases (depending on the environment) include:
- \(\mathrm{HCO_3^-}\) (bicarbonate ion)
- \(\mathrm{HSO_4^-}\) (hydrogen sulfate ion)
- \(\mathrm{H_2PO_4^-}\) (dihydrogen phosphate ion)
These amphoteric species are common in buffer systems and play essential roles in maintaining pH balance.
Example:
Identify how water acts in each of the following reactions:
▶️ Answer / Explanation
(a) \(\mathrm{HNO_3 + H_2O \rightarrow H_3O^+ + NO_3^-}\)
- Water accepts a proton → acts as a base.
(b) \(\mathrm{NH_3 + H_2O \rightleftharpoons NH_4^+ + OH^-}\)
- Water donates a proton → acts as an acid.
(c) \(\mathrm{2H_2O \rightleftharpoons H_3O^+ + OH^-}\)
- One molecule acts as an acid, the other as a base → self-ionization.
Final Note: Water’s amphoteric nature allows it to participate flexibly in acid–base equilibrium systems.
Conjugate Acid–Base Pairs and Their Relative Strengths
When an acid or base reacts with water, it undergoes ionization to form its conjugate base or conjugate acid. A conjugate acid–base pair consists of two species that differ by only one proton (\(\mathrm{H^+}\)). The relative strengths of acids and bases within a conjugate pair are inversely related a strong acid has a weak conjugate base, and a strong base has a weak conjugate acid.
1. Understanding Conjugate Acid–Base Pairs:
General representation: \(\mathrm{HA + H_2O \rightleftharpoons H_3O^+ + A^-}\)
![]()
- \(\mathrm{HA}\): Acid (proton donor)
- \(\mathrm{A^-}\): Conjugate base (after losing \(\mathrm{H^+}\))
- \(\mathrm{H_3O^+}\): Conjugate acid of water
Similarly, for a base reaction:
\(\mathrm{B + H_2O \rightleftharpoons BH^+ + OH^-}\)
- \(\mathrm{B}\): Base (proton acceptor)
- \(\mathrm{BH^+}\): Conjugate acid (after gaining \(\mathrm{H^+}\))
2. Relative Strength of Conjugate Pairs: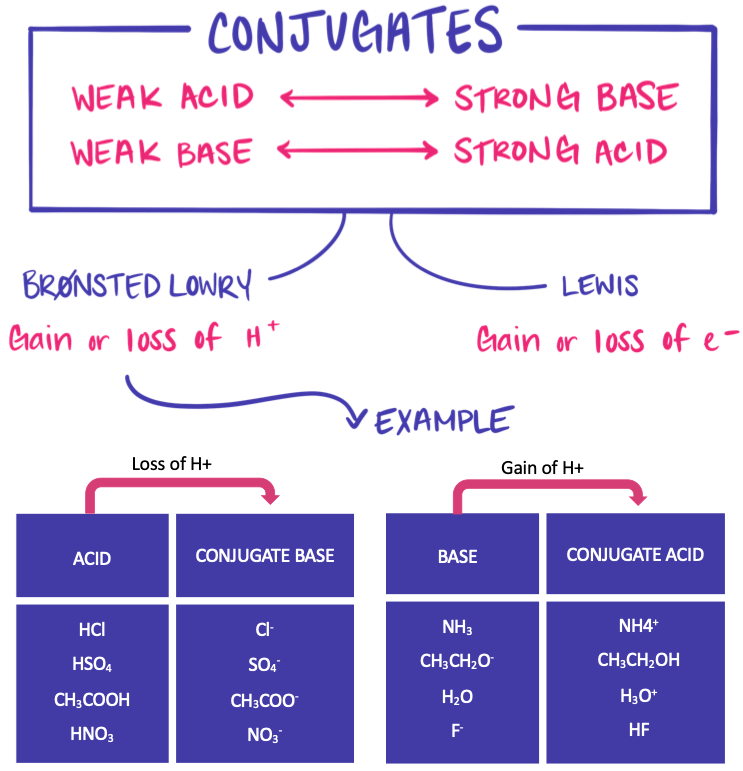
- The stronger the acid, the weaker its conjugate base.
- The stronger the base, the weaker its conjugate acid.
- This inverse relationship ensures equilibrium stability in acid–base systems.
Reason: A strong acid donates protons easily, leaving behind a conjugate base with very low tendency to accept a proton. Conversely, a weak acid has a conjugate base that more readily accepts protons.
3. Relationship Between Strengths of Conjugates:
For any acid–base pair in water:
\(\mathrm{K_a \times K_b = K_w}\)
- \(\mathrm{K_a}\): Acid dissociation constant
- \(\mathrm{K_b}\): Base dissociation constant of its conjugate base
- \(\mathrm{K_w}\): Ionization constant of water (\(1.0 \times 10^{-14}\) at 25°C)
Implication: If \(\mathrm{K_a}\) is large (strong acid), then \(\mathrm{K_b}\) is small (weak conjugate base), and vice versa.
4. Examples of Conjugate Acid–Base Pairs:
| Acid | Conjugate Base | Relative Strengths |
|---|---|---|
| \(\mathrm{HCl}\) | \(\mathrm{Cl^-}\) | Strong acid → Very weak base |
| \(\mathrm{H_2CO_3}\) | \(\mathrm{HCO_3^-}\) | Weak acid → Weak base |
| \(\mathrm{NH_4^+}\) | \(\mathrm{NH_3}\) | Weak acid → Moderate base |
| \(\mathrm{H_2O}\) | \(\mathrm{OH^-}\) | Amphoteric — acts as both acid and base |
5. Predicting Reaction Direction Using Conjugate Strengths:
- Proton transfer reactions proceed from the stronger acid–base pair to the weaker one.
- Thus, equilibrium favors formation of the weaker acid and weaker base.
Example: \(\mathrm{HF + NH_3 \rightleftharpoons NH_4^+ + F^-}\)
- \(\mathrm{HF}\) is a stronger acid than \(\mathrm{NH_4^+}\).
- Forward reaction favored; equilibrium shifts toward products.
Example:
For the reaction:
\(\mathrm{NH_3 + H_2O \rightleftharpoons NH_4^+ + OH^-}\)
Identify all conjugate acid–base pairs and compare their relative strengths.
▶️ Answer / Explanation
- Base: \(\mathrm{NH_3}\) → Conjugate acid: \(\mathrm{NH_4^+}\)
- Acid: \(\mathrm{H_2O}\) → Conjugate base: \(\mathrm{OH^-}\)
Relative strengths:
- \(\mathrm{NH_4^+}\) is a weak acid.
- \(\mathrm{OH^-}\) is a moderately strong base.
- Equilibrium lies toward the left (weaker species), meaning \(\mathrm{NH_3}\) and \(\mathrm{H_2O}\) predominate.
Final Note: Proton transfer reactions always favor the side with weaker acids and bases.