Oxidation-Reduction Rates
- Know that it is a redox reaction by a change in oxidation states
Oxidation States
- Oxidation Numbers: signifies the number of charges an atom would have in a molecule or ionic compound if electrons were completely transferred
- Know which atom has been reduced/oxidized based on the changes in their oxidation states
- Reduced = charge/oxidation state decreases (gains electrons); Oxidizing agent: electron acceptor
- Oxidized = charge/oxidation state increases (loses electrons); Reducing agent: electron donor
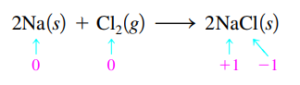
- Na is oxidized = reducing agent; chlorine is reduced = oxidizing agent
- Note: actual charges are written n+ or n-; oxidation states are written +n or –n
Oxidation State Rules
- Any element by itself: 0
- Monatomic ion = charge of ion
- Oxygen is usually -2 in its compounds
- Exception: peroxide (O₂²⁻) which is -1
- Hydrogen: +1
- Fluorine and the rest of the halogens are -1 (most of the time)
- Sulfur in SO4: +6
- Sum of oxidation states = 0 in compounds; sum of oxidation states = charge of the ion
- When don’t have rule for one of atoms/polyatomic ions, use the atom that does have a rule to find out oxidation state
- Ex:
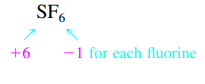
- Ex:
Balancing Oxidation-Reduction Equations
Using Oxidation Numbers
- Assign oxidation numbers to each atom
- Determine which atoms are being reduced and oxidized
- Write each half-reactions
- Balance elements
- For each half-reaction, balance charge using electrons
- Electrons must be on opposite sides and MUST have the same coefficients
- If necessary, multiply by integer to equalize electron count
- Add up half-reactions and write overall equation
- Balance remaining elements/compounds
Redox Reactions in Acidic Solutions vs Basic Solutions
Acidic Solutions
- Reaction involves H+ ions → For each half-reaction
- Balance all elements except H and O
- Balance oxygen using H2O
- Balance H using H+
- Balance the charge using electrons
Basic Solutions
- Reaction involves OH- ions → For each half-reaction
- Use the above method to obtain the final balanced equation as if H+ were present
- Add a number of OH- that is equal to the number of H+ ions to both sides of the equation
- We want to eliminate H+ by forming H2O
- Eliminate the number of H2O molecules that appear on both sides of the equation
- Check that the elements and charges are balanced
