Catalysis
- Catalyst: chemical agent that speeds up a reaction without being consumed by the reaction → can be used over and over again
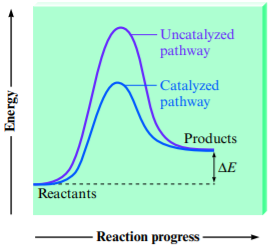
- Do not affect the free energy/enthalpy of a reaction!
- Reactions with a catalyst will typically require at least 2 steps
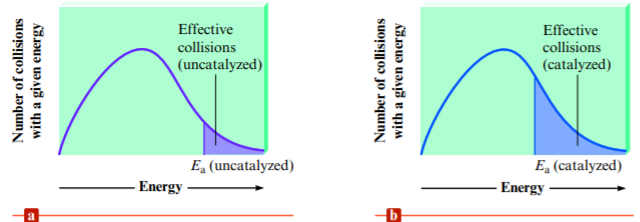
- Catalysts allow reactions to occur with a lower AE → lower activation energy means that more collisions will have enough energy to overcome AE and form a product → reaction rate increased
- Homogeneous catalyst: is present in the same phase as the reacting molecules
- Heterogeneous catalyst: exists in a different phase (usually as a solid; a catalytic converter is one type)
- 4 steps of heterogeneous catalysis
- Adsorption (collection of one substance on the surface of another substance) and activation of the react
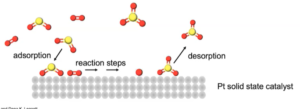 ants
ants - Migration of the adsorbed reactants on the surface
- Reaction of the adsorbed substances
- Escape (desorption) of the products
- Adsorption (collection of one substance on the surface of another substance) and activation of the react
How Catalysts Speed Up Reactions
- A catalyst increases the rate constant and lowers Ea Barrier by…
- Forming a more stable activated complex
- Increased collision frequency
- Improved orientation effects
- Allow chemical reactions to occur at lower temperatures
- Speeds up natural reactions ≠ cause them
- Reactions can occur without catalysts, but would be slower & cost a lot more energy
Activation Energy Barrier
|