AP Chemistry 5.2 Introduction to Rate Law Study Notes - New Syllabus Effective fall 2024
AP Chemistry 5.2 Introduction to Rate Law Study Notes- New syllabus
AP Chemistry 5.2 Introduction to Rate Law Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Represent experimental data with a consistent rate law expression.
Key Concepts:
- Measuring Reaction Rates Experimentally
- The Rate Law Expression
- Reaction Order and Overall Order
- The Rate Constant ( k)
- Determining Reaction Order from Initial Rates
Measuring Reaction Rates Experimentally
Experimental methods are used to monitor the change in the amount or concentration of reactants or products as a reaction proceeds. By measuring these changes over time, the rate of reaction can be determined.
Key Methods to Monitor Reactions:
| Method | Measured Quantity | Example |
|---|---|---|
| Change in gas volume | Collect volume of gas produced | \( \mathrm{H_2O_2 \rightarrow H_2O + O_2} \) |
| Change in pressure | Measure total gas pressure over time | \( \mathrm{2NO_2 \rightarrow 2NO + O_2} \) |
| Change in color (spectrophotometry) | Monitor absorbance using light intensity | \( \mathrm{Cr_2O_7^{2-} \rightarrow Cr^{3+}} \) |
| Change in conductivity | Detect ionic concentration changes | \( \mathrm{HCl + NaOH \rightarrow NaCl + H_2O} \) |
Reaction rate is determined by measuring how fast a measurable property (mass, pressure, color, etc.) changes over time, and converting that to a change in concentration.
Example
In the decomposition of hydrogen peroxide, \( \mathrm{2H_2O_2(aq) \rightarrow 2H_2O(l) + O_2(g)} \), the volume of oxygen collected at 25°C increased from \( \mathrm{0.0\,mL} \) to \( \mathrm{50.0\,mL} \) in \( \mathrm{100\,s.} \) Determine the average rate of oxygen production in \( \mathrm{mL/s.} \)
▶️ Answer / Explanation
Step 1: Average rate formula:
\( \mathrm{Rate = \dfrac{\Delta V_{O_2}}{\Delta t}} \)
Step 2: Substitute given values:
\( \mathrm{Rate = \dfrac{50.0 – 0.0}{100} = 0.50\,mL/s} \)
Result: The average rate of oxygen evolution is \( \mathrm{0.50\,mL/s.} \)
The Rate Law Expression
The rate law expresses the relationship between the rate of a chemical reaction and the concentrations of the reactants (and sometimes catalysts), each raised to a power determined experimentally.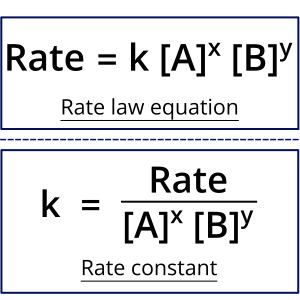
General Form:
\( \mathrm{Rate = k[A]^m[B]^n} \)
- \( \mathrm{k} \): rate constant
- \( \mathrm{[A], [B]} \): molar concentrations of reactants
- \( \mathrm{m, n} \): reaction orders (determined experimentally)
The rate law shows how changing reactant concentrations affects the reaction rate — it cannot be deduced from the balanced equation alone but must be found experimentally.
Example
The experimental rate law for the reaction \( \mathrm{2NO + O_2 \rightarrow 2NO_2} \) is found to be \( \mathrm{Rate = k[NO]^2[O_2]} \). What does this rate law indicate about how each reactant affects the rate?
▶️ Answer / Explanation
- The reaction is second order with respect to \( \mathrm{NO} \) — doubling \( \mathrm{[NO]} \) increases rate by \( 2^2 = 4 \) times.
- The reaction is first order with respect to \( \mathrm{O_2} \) — doubling \( \mathrm{[O_2]} \) doubles the rate.
- Overall order: \( 2 + 1 = 3 \) (third-order reaction).
Reaction Order and Overall Order
The order of a reaction with respect to a reactant is the power to which its concentration is raised in the rate law. The overall order is the sum of all individual orders.
General Form: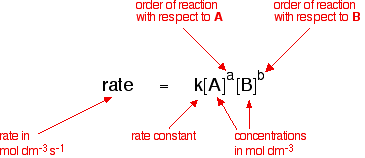
For \( \mathrm{Rate = k[A]^m[B]^n} \):
- Order with respect to \( \mathrm{A} = m \)
- Order with respect to \( \mathrm{B} = n \)
- Overall order \( = m + n \)
Reaction order indicates how sensitive the rate is to concentration changes for each reactant and helps determine the reaction mechanism.
Example
For the reaction \( \mathrm{A + 2B \rightarrow C} \), the rate law is \( \mathrm{Rate = k[A]^1[B]^2.} \) Determine:
- (a) The order with respect to \( \mathrm{A} \)
- (b) The order with respect to \( \mathrm{B} \)
- (c) The overall order of the reaction
▶️ Answer / Explanation
Step 1: Identify exponents in rate law.
- \( \mathrm{m = 1} \) → first order in \( \mathrm{A} \)
- \( \mathrm{n = 2} \) → second order in \( \mathrm{B} \)
Step 2: Add to find overall order.
\( \mathrm{Overall\ order = 1 + 2 = 3} \)
Result: The reaction is first order in \( \mathrm{A} \), second order in \( \mathrm{B} \), and third order overall.
The Rate Constant ( k)
The rate constant (k) is the proportionality constant in the rate law that relates reactant concentrations to the rate of reaction. Its value depends on temperature and the specific reaction mechanism.
Key Characteristics:
- \( \mathrm{k} \) is constant for a given reaction at a fixed temperature.
- \( \mathrm{k} \) increases with increasing temperature.
- The units of \( \mathrm{k} \) depend on the overall order of the reaction.
Units of Rate Constant:
| Overall Order | Units of \( \mathrm{k} \) |
|---|---|
| Zero order | \( \mathrm{mol\,L^{-1}\,s^{-1}} \) |
| First order | \( \mathrm{s^{-1}} \) |
| Second order | \( \mathrm{L\,mol^{-1}\,s^{-1}} \) |
| Third order | \( \mathrm{L^2\,mol^{-2}\,s^{-1}} \) |
The rate constant provides a quantitative measure of how fast a reaction proceeds at a given temperature.
Example
For the reaction \( \mathrm{A \rightarrow Products} \), the rate is \( \mathrm{1.5\times10^{-3}\,mol\,L^{-1}\,s^{-1}} \) when \( \mathrm{[A] = 0.30\,mol\,L^{-1}} \). If the reaction is first order, calculate the rate constant \( \mathrm{k.} \)
▶️ Answer / Explanation
Step 1: Use rate law: \( \mathrm{Rate = k[A]} \)
Step 2: Substitute values:
\( \mathrm{1.5\times10^{-3} = k(0.30)} \)
Step 3: Solve for \( \mathrm{k:} \)
\( \mathrm{k = \dfrac{1.5\times10^{-3}}{0.30} = 5.0\times10^{-3}\,s^{-1}} \)
Result: \( \mathrm{k = 5.0\times10^{-3}\,s^{-1}} \)
Determining Reaction Order from Initial Rates
The method of initial rates determines the order of a reaction by comparing how the initial rate changes when the initial concentrations of reactants are varied.
Procedure:
- Perform several experiments with varying initial reactant concentrations.
- Measure the initial rate for each experiment.
- Determine how the rate changes when each reactant’s concentration changes.
Key Relation:
\( \mathrm{\dfrac{Rate_2}{Rate_1} = \left(\dfrac{[A]_2}{[A]_1}\right)^m \left(\dfrac{[B]_2}{[B]_1}\right)^n} \)
- \( \mathrm{m} \) and \( \mathrm{n} \): reaction orders determined experimentally.
By holding one reactant constant and varying the other, the order with respect to each reactant can be found from the rate ratio.
Example
Using Initial Rates to Determine Reaction Order , The following data were collected for the reaction \( \mathrm{A + B \rightarrow Products} \):
| Experiment | \( \mathrm{[A]\,(mol/L)} \) | \( \mathrm{[B]\,(mol/L)} \) | Initial Rate (\( \mathrm{mol\,L^{-1}\,s^{-1}} \)) |
|---|---|---|---|
| 1 | 0.10 | 0.10 | 0.020 |
| 2 | 0.20 | 0.10 | 0.040 |
| 3 | 0.10 | 0.20 | 0.080 |
▶️ Answer / Explanation
Step 1: Compare Experiments 1 and 2 (B constant):
\( \mathrm{\dfrac{Rate_2}{Rate_1} = \left(\dfrac{[A]_2}{[A]_1}\right)^m} \)
\( \mathrm{\dfrac{0.040}{0.020} = \left(\dfrac{0.20}{0.10}\right)^m \Rightarrow 2 = 2^m \Rightarrow m = 1} \)
Step 2: Compare Experiments 1 and 3 (A constant):
\( \mathrm{\dfrac{0.080}{0.020} = \left(\dfrac{0.20}{0.10}\right)^n \Rightarrow 4 = 2^n \Rightarrow n = 2} \)
Step 3: Write the rate law:
\( \mathrm{Rate = k[A]^1[B]^2} \)
Step 4: Determine overall order:
Overall order \( = 1 + 2 = 3 \)
Result: The reaction is first order in \( \mathrm{A} \), second order in \( \mathrm{B} \), and third order overall.
