AP Chemistry 5.5 Collision Model Study Notes - New Syllabus Effective fall 2024
AP Chemistry 5.5 Collision Model Study Notes- New syllabus
AP Chemistry 5.5 Collision Model Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship between the rate of an elementary reaction and the frequency, energy, and orientation of particle collisions.
Key Concepts:
- Successful Collisions and Product Formation
- Energy and Orientation in Successful Collisions
Successful Collisions and Product Formation
For an elementary reaction to successfully form products, the reacting particles must collide effectively. An effective (successful) collision is one that has the right combination of energy and orientation to initiate bond breaking and bond formation during the reaction.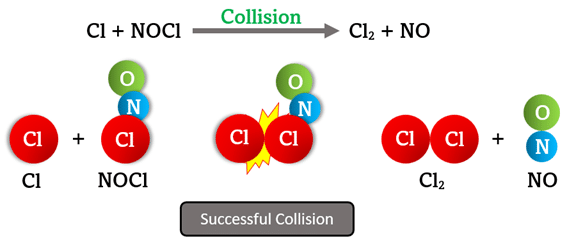
Collision Theory Concept:
- Chemical reactions occur when molecules collide with one another.
- However, not all collisions result in a reaction.
- Only collisions that meet the necessary energy threshold and geometric orientation can form products.
Key Idea: A reaction only proceeds if the reactant molecules collide with enough kinetic energy to overcome the activation energy barrier and in a proper alignment that allows old bonds to break and new bonds to form.
Illustration — Collision Process: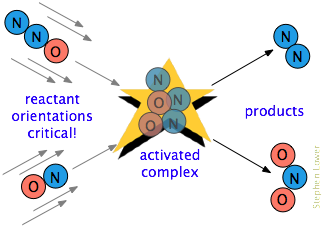
- Step 1: Reactants approach each other and collide.
- Step 2: If energy ≥ activation energy, temporary activated complex (transition state) forms.
- Step 3: Bonds rearrange → new products form.
\( \mathrm{A + B \rightarrow [AB]^* \rightarrow Products} \)
- \( \mathrm{[AB]^*} \): Activated complex (unstable, short-lived intermediate).
Example
In the reaction \( \mathrm{Cl + NOCl \rightarrow Cl_2 + NO} \), what must occur for the collision between \( \mathrm{Cl} \) and \( \mathrm{NOCl} \) to successfully produce \( \mathrm{Cl_2} \) and \( \mathrm{NO}? \)
▶️ Answer / Explanation
Step 1: The \( \mathrm{Cl} \) atom must collide with \( \mathrm{NOCl} \) with sufficient energy to break the \( \mathrm{Cl–N} \) bond in \( \mathrm{NOCl.} \)
Step 2: The collision must occur at the correct site — the chlorine end of \( \mathrm{NOCl} \) — so that a new \( \mathrm{Cl–Cl} \) bond can form.
Step 3: The activated complex \( \mathrm{[Cl–NO–Cl]^*} \) forms briefly, then rearranges to produce \( \mathrm{Cl_2} \) and \( \mathrm{NO.} \)
Result: A successful collision requires both:
- Sufficient kinetic energy (≥ activation energy)
- Correct molecular orientation for bond rearrangement
Energy and Orientation in Successful Collisions
In most chemical reactions, only a small fraction of molecular collisions lead to product formation. A collision is successful only if the reacting molecules collide with both:
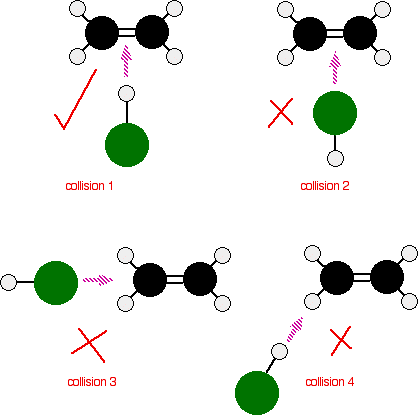
- Sufficient kinetic energy to overcome the activation energy barrier, and
- Proper orientation that allows the necessary bonds to break and new bonds to form.
Key Concept — Activation Energy (\( \mathrm{E_a} \)):
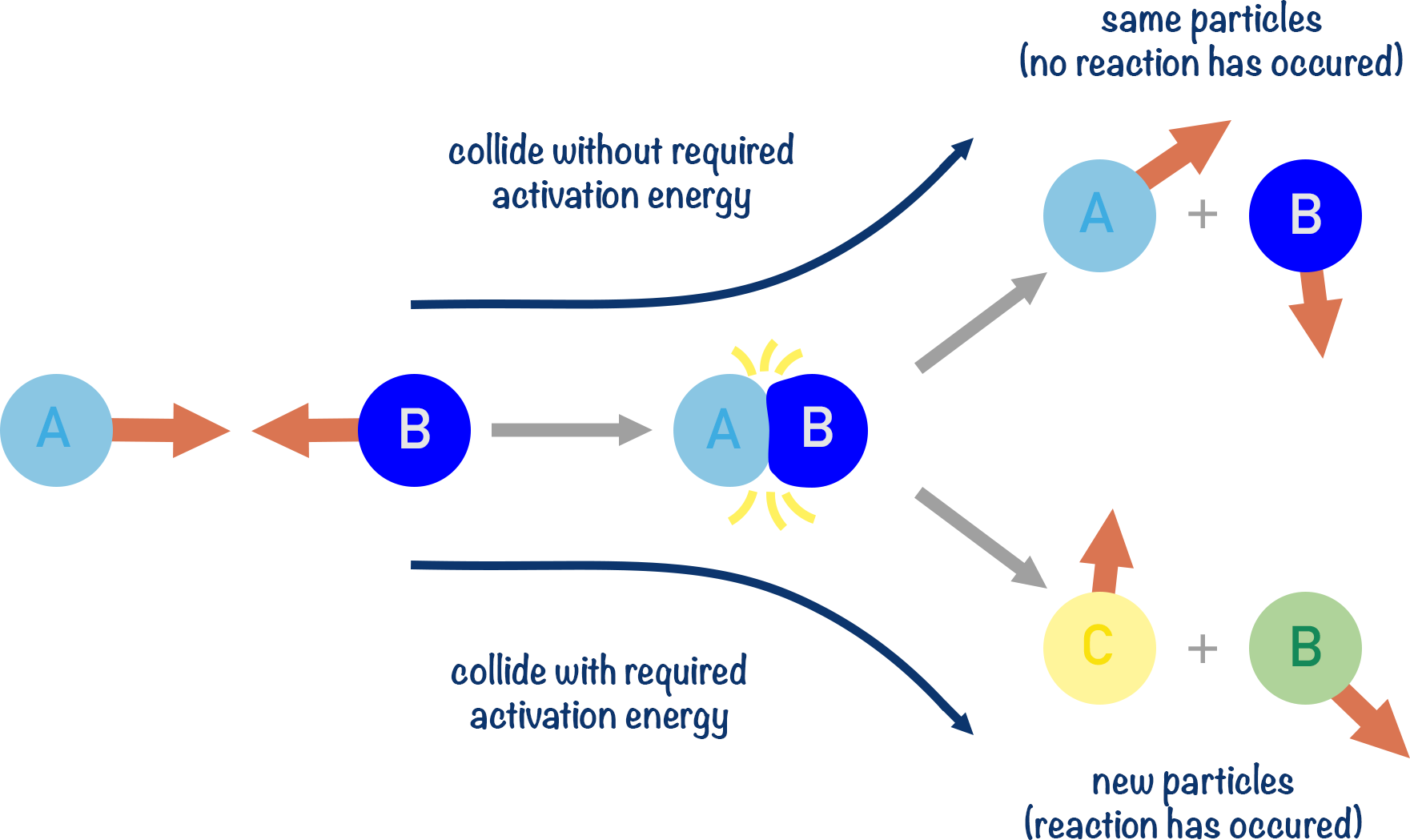
- \( \mathrm{E_a} \) is the minimum energy that reactant particles must possess for a reaction to occur.
- Particles with energy < \( \mathrm{E_a} \) will simply bounce off each other — no reaction occurs.
- Particles with energy ≥ \( \mathrm{E_a} \) can form an activated complex (transition state) and proceed to products.
\( \mathrm{A + B \xrightarrow{collision} [AB]^* \rightarrow Products} \)
Orientation Factor:
- Even if particles have enough energy, a wrong orientation can prevent reaction.
- Reactants must collide in a way that brings the correct atoms into contact for bond rearrangement.
Key Idea: A collision leads to reaction only when it satisfies both conditions: (1) energy ≥ \( \mathrm{E_a} \) and (2) correct orientation. Hence, only a small fraction of total collisions actually form products.
Example
The gas-phase reaction \( \mathrm{NO + O_3 \rightarrow NO_2 + O_2} \) occurs slowly at room temperature. Explain why most collisions between \( \mathrm{NO} \) and \( \mathrm{O_3} \) molecules do not result in reaction.
▶️ Answer / Explanation
Step 1: Consider energy requirement.
At room temperature, most \( \mathrm{NO} \) and \( \mathrm{O_3} \) molecules move too slowly to overcome the activation energy barrier required to break the \( \mathrm{O–O} \) bond in ozone.
Step 2: Consider molecular orientation.
Even when energy is sufficient, the molecules must collide so that the oxygen atom of \( \mathrm{O_3} \) is properly aligned to bond with nitrogen in \( \mathrm{NO.} \) Incorrect geometry leads to ineffective collisions.
Step 3: Combine both effects.
Only a small percentage of collisions satisfy both conditions (sufficient energy + correct orientation), so the overall reaction rate is low.
Result: Most \( \mathrm{NO–O_3} \) collisions are ineffective because they lack the required kinetic energy or alignment. Only those few that meet both criteria lead to product formation.
Maxwell–Boltzmann Energy Distribution and Temperature Dependence
The Maxwell–Boltzmann distribution describes how the kinetic energies of particles in a system are distributed at a given temperature. It shows that not all particles have the same energy — most have moderate energy, some have low energy, and a few have very high energy.
This distribution helps estimate the fraction of molecules that have enough energy to overcome the activation energy barrier (\( \mathrm{E_a} \)) and successfully react.
Shape of the Maxwell–Boltzmann Curve
The curve plots the number of particles (y-axis) versus their kinetic energy (x-axis).
![]()
- It starts at zero, rises to a peak, and then gradually falls — forming an asymmetric bell-shaped curve.
- The peak represents the most probable energy of particles.
- The area under the curve represents the total number of molecules, which remains constant regardless of temperature.
- The shaded area to the right of \( \mathrm{E_a} \) represents the fraction of molecules with enough energy to react.
Effect of Temperature Increase:
- When temperature increases:
- The curve flattens and shifts right, meaning average particle energy increases.
- The number of high-energy molecules (with \( \mathrm{E \geq E_a} \)) increases significantly.
- This results in a higher reaction rate, even though total particle number remains the same.
Qualitative Comparison:
| Condition | Curve Shape | Fraction of Particles with \( \mathrm{E \geq E_a} \) | Reaction Rate |
|---|---|---|---|
| Lower Temperature | Narrow, tall peak | Small shaded area | Slower |
| Higher Temperature | Broader, lower peak shifted right | Large shaded area beyond \( \mathrm{E_a} \) | Faster |
Mathematical Implication (Arrhenius Equation):
\( \mathrm{k = A e^{-\dfrac{E_a}{RT}}} \)
- As temperature \( \mathrm{T} \) increases, \( \mathrm{e^{-\dfrac{E_a}{RT}}} \) increases → larger \( \mathrm{k} \) → faster reaction.
- Even a small rise in temperature can greatly increase the number of molecules with \( \mathrm{E \geq E_a.} \)
Example
The decomposition of \( \mathrm{N_2O_5} \) has an activation energy of \( \mathrm{100\,kJ/mol.} \) Explain qualitatively, using the Maxwell–Boltzmann distribution, why heating the system from \( \mathrm{300\,K} \) to \( \mathrm{330\,K} \) causes a large increase in reaction rate.
▶️ Answer / Explanation
Step 1: At \( \mathrm{300\,K} \), only a small fraction of \( \mathrm{N_2O_5} \) molecules have energy ≥ \( \mathrm{E_a = 100\,kJ/mol.} \)
Step 2: Increasing temperature to \( \mathrm{330\,K} \) shifts the Maxwell–Boltzmann curve to higher energies and flattens it.
→ The shaded area beyond \( \mathrm{E_a} \) becomes much larger.
Step 3: More molecules now have enough kinetic energy to overcome \( \mathrm{E_a} \), resulting in more effective collisions per second.
Step 4: As a result, \( \mathrm{k} \) increases exponentially according to the Arrhenius relation, and the reaction rate increases substantially.
Result: Even a moderate increase in temperature greatly increases the fraction of molecules with sufficient energy, making the reaction proceed much faster.
