AP Chemistry 5.8 Reaction Mechanism and Rate Law Study Notes - New Syllabus Effective fall 2024
AP Chemistry 5.8 Reaction Mechanism and Rate Law Study Notes.- New syllabus
AP Chemistry 5.8 Reaction Mechanism and Rate Law Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Identify the rate law for a reaction from a mechanism in which the first step is rate limiting.
Key Concepts:
- Rate Law from a Mechanism with a Rate-Limiting Step
Rate Law from a Mechanism with a Rate-Limiting Step
In a multi-step reaction mechanism, the rate law is determined by the slowest elementary step, known as the rate-limiting step. This step controls the overall reaction rate because the subsequent (faster) steps cannot proceed more quickly than this slowest step allows.
- Each elementary step has its own molecularity that defines its individual rate law.
- If the first step is slow (rate-limiting) and irreversible, the overall rate law is written directly from the reactants in that step.
- The rate of the overall reaction depends only on the species involved in the rate-determining step (RDS).
Key Idea: The rate-limiting step acts as a bottleneck for the entire reaction. Its molecularity (the number of reacting species in that step) directly determines the form of the overall rate law.
General Expression:
If Step 1 is the rate-limiting step, \( \mathrm{Rate = k [Reactant_1]^a [Reactant_2]^b} \)
- \( \mathrm{k} \): rate constant for the slow step
- \( \mathrm{a, b} \): stoichiometric coefficients of the reactants in the slow step
Important Notes:
- Only the reactants in the rate-determining step appear in the rate law.
- Species involved in faster steps (including intermediates) do not affect the rate law if the first step is slow.
- When the first step is slow, the mechanism is straightforward — no equilibrium relationships or substitutions are needed.
Example
The following mechanism is proposed for the oxidation of carbon monoxide by nitrogen dioxide:
Step 1 (slow): \( \mathrm{NO_2 + NO_2 \rightarrow NO + NO_3} \)
Step 2 (fast): \( \mathrm{NO_3 + CO \rightarrow NO_2 + CO_2} \)
What is the rate law for the overall reaction?
▶️ Answer / Explanation
Step 1: The slow step (Step 1) controls the rate of the overall reaction.
Step 2: Write rate law from Step 1:
\( \mathrm{Rate = k[NO_2]^2} \)
Step 3: Step 2 is fast and does not influence the rate law.
Step 4: Verify that the mechanism yields the correct overall equation: \( \mathrm{NO_2 + CO \rightarrow NO + CO_2} \).
Result: The rate law is \( \mathrm{Rate = k[NO_2]^2} \), determined solely by the molecularity of the rate-limiting step.
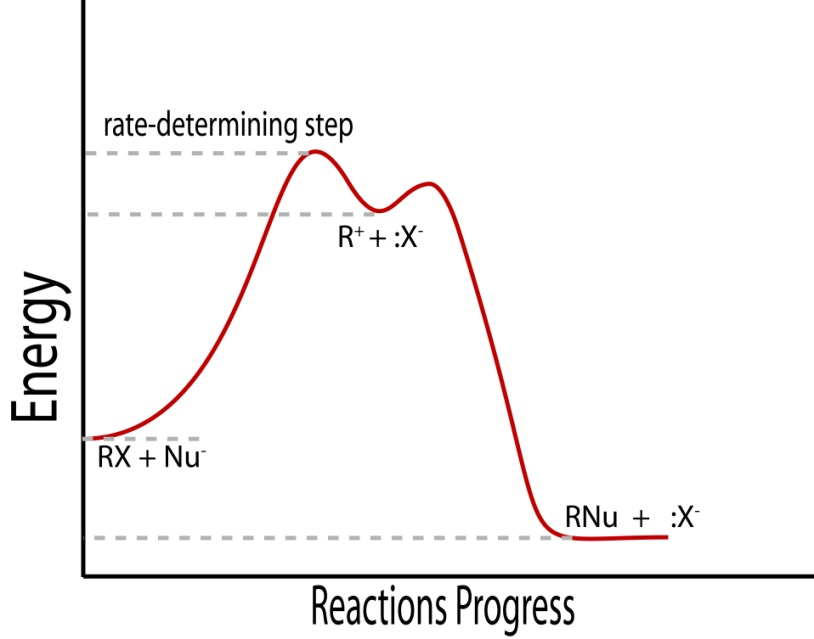
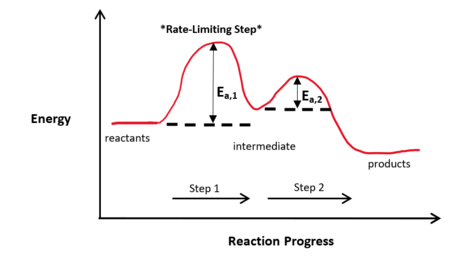
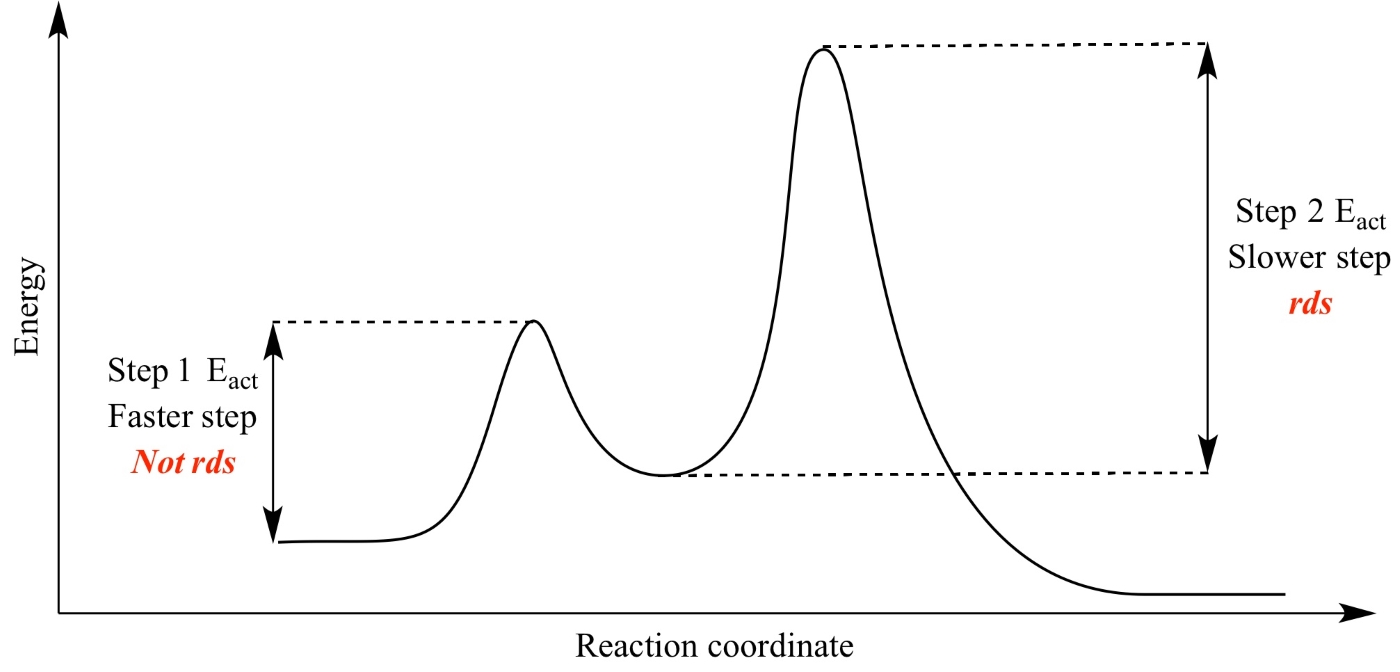
The Rate-Limiting Step Concept
| Step Type | Effect on Rate Law | Notes |
|---|---|---|
| Slow (Rate-Determining Step) | Determines the overall rate law | Acts as the bottleneck of the mechanism |
| Fast Step | Does not appear in rate law (unless reversible equilibrium exists) | Occurs after the rate-limiting step |
| First Step Slow | Rate law equals molecularity of first step | No intermediates appear in the rate law |
When the first step of a reaction mechanism is the rate-limiting step, the rate law is written directly from the reactants and stoichiometry of that step, since it determines how fast the overall reaction can proceed.
Example
The decomposition of hydrogen peroxide (\( \mathrm{H_2O_2} \)) in the presence of iodide ion (\( \mathrm{I^-} \)) proceeds by the following mechanism:
Step 1 (slow): \( \mathrm{H_2O_2 + I^- \rightarrow H_2O + IO^-} \)
Step 2 (fast): \( \mathrm{H_2O_2 + IO^- \rightarrow H_2O + O_2 + I^-} \)
Determine the overall rate law for the reaction.
▶️ Answer / Explanation
Step 1: Identify the rate-limiting step.
The first step is labeled as slow, so it controls the rate of the entire reaction.
Step 2: Write the rate law for the slow step.
\( \mathrm{Rate = k[H_2O_2][I^-]} \)
Step 3: Check the role of intermediates.
- The intermediate \( \mathrm{IO^-} \) forms in Step 1 and is consumed in Step 2.
- Since the slow step determines the rate, \( \mathrm{IO^-} \) does not appear in the rate law.
Step 4: Verify the overall equation.
Adding both steps:
\( \mathrm{2H_2O_2 \rightarrow 2H_2O + O_2} \)
Step 5: Interpret the result.
The rate law depends only on the concentrations of \( \mathrm{H_2O_2} \) and \( \mathrm{I^-} \) involved in the slow (first) step.
Result: \( \mathrm{Rate = k[H_2O_2][I^-]} \)
Explanation: The first step is slow and determines how quickly the reaction proceeds. The presence of iodide acts as a catalyst — it participates in the mechanism but is regenerated, so it does not appear in the overall reaction.