AP Chemistry 5.9 Steady State Approximation Study Notes - New Syllabus Effective fall 2024
AP Chemistry 5.9 Steady State Approximation Study Notes.- New syllabus
AP Chemistry 5.9 Steady State Approximation Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Identify the rate law for a reaction from a mechanism in which the first step is not rate limiting.
Key Concepts:
- Rate Law from a Mechanism in Which the First Step Is Not Rate-Limiting
Rate Law from a Mechanism in Which the First Step Is Not Rate-Limiting
When the first step of a reaction mechanism is fast and reversible, and a later step is slow (rate-limiting), the overall rate law cannot be written directly from the reactants in the slow step. Instead, we must use the pre-equilibrium approximation to relate intermediates back to the initial reactants and derive the correct rate law.
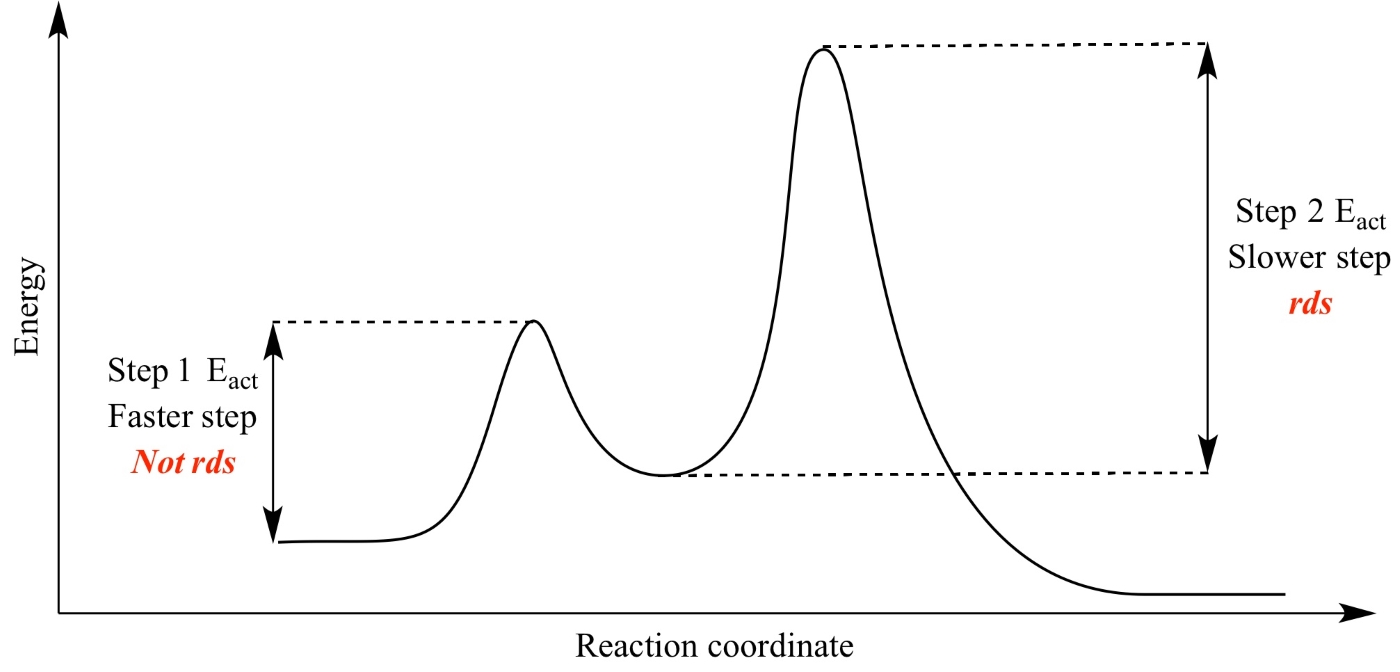
- If the first step is fast and reversible, it quickly establishes a dynamic equilibrium between its reactants and products.
- The slow step determines the overall rate, but its rate law may include intermediates formed in the first (fast) step.
- We must eliminate intermediates from the rate law by substituting expressions derived from the equilibrium condition of the first step.
When the first step is not rate-limiting, the system reaches pre-equilibrium — a temporary balance between forward and reverse rates in the fast step. We use this condition to express the concentration of intermediates in terms of known reactant concentrations.
Pre-Equilibrium Approximation:
For a fast, reversible first step: \( \mathrm{A + B \rightleftharpoons C} \) followed by a slow step: \( \mathrm{C + D \rightarrow \text{Products}} \)
- From the fast equilibrium step, the equilibrium constant is \( \mathrm{K = \dfrac{[C]}{[A][B]}} \)
- Rearrange to express the intermediate \( \mathrm{[C]} \): \( \mathrm{[C] = K[A][B]} \)
- Substitute into the rate law for the slow step: \( \mathrm{Rate = k[C][D] = kK[A][B][D]} \)
- Hence, the overall rate law is \( \mathrm{Rate = k_{obs}[A][B][D]} \), where \( \mathrm{k_{obs} = kK} \) (observed rate constant).
Summary — When the First Step Is Not Rate-Limiting
| Condition | Feature | Effect on Rate Law |
|---|---|---|
| First Step Fast and Reversible | Establishes pre-equilibrium | Intermediate concentration found using equilibrium constant |
| Second Step Slow | Controls overall rate | Rate law combines equilibrium and slow-step relations |
| Overall Effect | Eliminate intermediates via substitution | Final rate law depends only on observable reactants |
Example
The reaction between nitric oxide and bromine proceeds by the following mechanism:
Step 1 (fast, reversible): \( \mathrm{NO + Br_2 \rightleftharpoons NOBr_2} \)
Step 2 (slow): \( \mathrm{NOBr_2 + NO \rightarrow 2NOBr} \)
Determine the overall rate law for the reaction.
▶️ Answer / Explanation
Step 1: The slow (rate-limiting) step is Step 2.
\( \mathrm{Rate = k_2[NOBr_2][NO]} \)
Step 2: From Step 1 (fast equilibrium), write the equilibrium constant:
\( \mathrm{K = \dfrac{[NOBr_2]}{[NO][Br_2]}} \) → \( \mathrm{[NOBr_2] = K[NO][Br_2]} \)
Step 3: Substitute this into the rate law of the slow step:
\( \mathrm{Rate = k_2(K[NO][Br_2])[NO] = k_2K[NO]^2[Br_2]} \)
Step 4: Simplify using the observed rate constant:
\( \mathrm{Rate = k_{obs}[NO]^2[Br_2]} \), where \( \mathrm{k_{obs} = k_2K} \)
Result: The overall rate law is \( \mathrm{Rate = k_{obs}[NO]^2[Br_2]} \). This agrees with experimental data, confirming the mechanism’s validity.
Example
The following mechanism is proposed for the reaction between hydrogen and iodine monochloride:
Step 1 (fast, reversible): \( \mathrm{ICl + H_2 \rightleftharpoons HCl + HI} \)
Step 2 (slow): \( \mathrm{HI + ICl \rightarrow I_2 + HCl} \)
Determine the overall rate law for the reaction, assuming the first step is fast and reversible.
▶️ Answer / Explanation
Step 1: The rate-determining step is Step 2.
\( \mathrm{Rate = k_2[HI][ICl]} \)
Step 2: From Step 1 (fast equilibrium), write the equilibrium constant:
\( \mathrm{K = \dfrac{[HCl][HI]}{[ICl][H_2]}} \)
Step 3: Rearrange to express \( \mathrm{[HI]} \):
\( \mathrm{[HI] = K\dfrac{[ICl][H_2]}{[HCl]}} \)
Step 4: Substitute into the slow-step rate law:
\( \mathrm{Rate = k_2[K\dfrac{[ICl][H_2]}{[HCl]}][ICl] = k_2K\dfrac{[ICl]^2[H_2]}{[HCl]}} \)
Step 5: Simplify the expression:
\( \mathrm{Rate = k_{obs}\dfrac{[ICl]^2[H_2]}{[HCl]}} \), where \( \mathrm{k_{obs} = k_2K} \)
Result: The overall rate law is \( \mathrm{Rate = k_{obs}\dfrac{[ICl]^2[H_2]}{[HCl]}} \). Increasing \( \mathrm{[HCl]} \) slows the reaction, consistent with the pre-equilibrium model.
