AP Chemistry 6.3 Heat Transfer and Thermal Equilibrium Study Notes - New Syllabus Effective fall 2024
AP Chemistry 6.3 Heat Transfer and Thermal Equilibrium Study Notes.- New syllabus
AP Chemistry 6.3 Heat Transfer and Thermal Equilibrium Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship between the transfer of thermal energy and molecular collisions.
Key Concepts:
- Relationship Between Temperature and Average Kinetic Energy
- Heat Transfer Through Particle Collisions
- Thermal Equilibrium and Equal Average Kinetic Energy
Relationship Between Temperature and Average Kinetic Energy
Temperature is a direct measure of the average kinetic energy of the particles in a substance. A warmer object has particles moving faster, on average, than those in a cooler object. This difference in kinetic energy explains why energy flows as heat between substances at different temperatures.
Definition: The average kinetic energy of particles in a sample is proportional to its absolute temperature (in kelvin):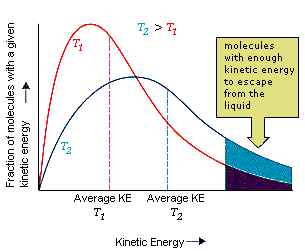
\( \mathrm{KE_{avg} = \dfrac{3}{2}RT} \)
- \( \mathrm{KE_{avg}} \): average kinetic energy per mole of gas particles
- \( \mathrm{R} \): universal gas constant (\( \mathrm{8.314\ J\ mol^{-1}\ K^{-1}} \))
- \( \mathrm{T} \): absolute temperature (K)
Key Relationships:
- When \( \mathrm{T} \) increases, the average kinetic energy \( \mathrm{KE_{avg}} \) also increases proportionally.
- When temperature doubles (in kelvin), the average kinetic energy also doubles.
- At 0 K (absolute zero), all molecular motion theoretically stops — the lowest possible energy state.
Key Idea: The temperature of a system is a direct measure of the average kinetic energy of its particles. Thus, a higher temperature means faster-moving particles and greater molecular motion.
| Quantity | Symbol | Meaning | Relationship to Temperature |
|---|---|---|---|
| Average Kinetic Energy | \( \mathrm{KE_{avg}} \) | Energy of particle motion | \( \mathrm{KE_{avg} \propto T} \) |
| Absolute Temperature | \( \mathrm{T} \) | Measure of average kinetic energy | Higher \( \mathrm{T} \) → higher \( \mathrm{KE_{avg}} \) |
| Absolute Zero | 0 K | All molecular motion ceases | Minimum \( \mathrm{KE_{avg}} = 0 \) |
Example:
Two identical containers hold equal numbers of gas molecules — one at 300 K and the other at 600 K. Compare their average kinetic energies and molecular speeds.
▶️ Answer/Explanation
Step 1: The average kinetic energy is proportional to temperature: \( \mathrm{KE_{avg} \propto T} \).
Step 2: \( \mathrm{\dfrac{KE_{2}}{KE_{1}} = \dfrac{T_2}{T_1} = \dfrac{600}{300} = 2} \).
Step 3: Therefore, the average kinetic energy at 600 K is twice as large as at 300 K.
Step 4: The root-mean-square speed (\( \mathrm{v_{rms}} \)) of gas molecules is given by \( \mathrm{v_{rms} = \sqrt{\dfrac{3RT}{M}}} \). Thus, \( \mathrm{v_{rms}} \propto \sqrt{T} \).
Step 5: \( \mathrm{\dfrac{v_{2}}{v_{1}} = \sqrt{\dfrac{T_2}{T_1}} = \sqrt{2} \approx 1.41} \).
Final Answer: At 600 K, the molecules have twice the average kinetic energy and move about 1.4 times faster than at 300 K.
Heat Transfer Through Particle Collisions
When two bodies at different temperatures come into contact, energy is transferred from the warmer body to the cooler one. This energy transfer occurs through the collisions of particles and is referred to as heat transfer, heat exchange, or transfer of energy as heat.
Heat transfer is the process by which energy is exchanged between substances due to a difference in temperature. The energy flows spontaneously from the region of higher average kinetic energy (hotter) to the region of lower average kinetic energy (cooler).
\( \mathrm{q = m c \Delta T} \) 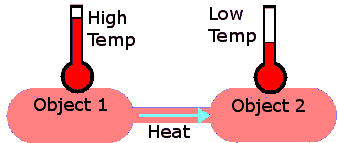
- \( \mathrm{q} \): energy transferred as heat (in joules)
- \( \mathrm{m} \): mass of the substance (in grams)
- \( \mathrm{c} \): specific heat capacity (in \( \mathrm{J\ g^{-1}\ ^\circ C^{-1}} \))
- \( \mathrm{\Delta T} \): temperature change (\( \mathrm{T_{final} – T_{initial}} \))
Microscopic Description:
- Particles in the warmer body move faster (higher kinetic energy).
- When they collide with slower particles in the cooler body, some kinetic energy is transferred.
- Over time, as these collisions continue, the average kinetic energy of both systems changes until equilibrium is reached.
Key Relationships:
- Energy always flows spontaneously from hot → cold until equilibrium.
- The total energy of the two systems is conserved (\( \mathrm{q_{lost} = -q_{gained}} \)).
- The amount of energy transferred depends on mass, specific heat, and temperature change of each body.
Heat transfer occurs because of collisions between particles. Faster-moving particles (warmer body) transfer energy to slower-moving ones (cooler body) until both reach the same average kinetic energy and temperature.
| Term | Symbol / Equation | Meaning | Example |
|---|---|---|---|
| Heat Transfer | \( \mathrm{q} \) | Energy transferred due to temperature difference | Heat flows from hot metal to cool water |
| Energy Conservation | \( \mathrm{q_{lost} = -q_{gained}} \) | Total energy remains constant | Metal cools while water warms |
| Specific Heat Capacity | \( \mathrm{c} \) | Amount of heat needed to raise 1 g by 1 °C | Water: \( \mathrm{4.18\ J\ g^{-1}\ ^\circ C^{-1}} \) |
Example :
A 100 g piece of copper at 95 °C is placed into 200 g of water at 25 °C. The final temperature of both substances is 30 °C. Assuming no heat loss to the surroundings, calculate the amount of heat transferred by each substance.
▶️ Answer / Explanation
Step 1: Use \( \mathrm{q = m c \Delta T} \).
Step 2: For copper: \( \mathrm{m = 100\ g, \ c = 0.385\ J\ g^{-1}\ ^\circ C^{-1}, \ \Delta T = (30 – 95) = -65\ ^\circ C} \). \( \mathrm{q_{Cu} = 100(0.385)(-65) = -2502.5\ J} \).
Step 3: For water: \( \mathrm{m = 200\ g, \ c = 4.18\ J\ g^{-1}\ ^\circ C^{-1}, \ \Delta T = (30 – 25) = 5\ ^\circ C} \). \( \mathrm{q_{H_2O} = 200(4.18)(5) = 4180\ J} \).
Step 4: Energy lost by copper ≈ energy gained by water (minor difference due to rounding): \( \mathrm{q_{Cu} + q_{H_2O} \approx 0} \).
Step 5: Energy flows from hot copper → cool water until both reach the same final temperature.
Final Answer: \( \mathrm{q_{lost, Cu} = -2.50\ kJ} \), \( \mathrm{q_{gained, H_2O} = +2.50\ kJ} \). Heat transfer continues until both are at 30 °C.
Example :
Explain on a particle level what happens when an ice cube is placed in a cup of warm water.
▶️ Answer / Explanation
Step 1: Water molecules in the warm liquid move rapidly (high kinetic energy), while the particles in ice vibrate slowly (low kinetic energy).
Step 2: As they collide at the interface, energy is transferred from water molecules to the ice molecules.
Step 3: The ice absorbs energy, causing bonds to break and melting to occur.
Step 4: As energy leaves the water, it cools slightly until the system reaches thermal equilibrium.
Final Answer: Heat transfer through collisions equalizes the average kinetic energy of all particles — ice melts, water cools, and the final temperature stabilizes.
Thermal Equilibrium and Equal Average Kinetic Energy
When two substances at different temperatures are placed in contact, they exchange energy through collisions of their particles. Over time, the transfer of energy continues until the two substances reach the same temperature. At this point, the system is said to have reached thermal equilibrium.
Thermal equilibrium is the condition in which two bodies in thermal contact have reached the same temperature, meaning their particles have the same average kinetic energy and no net energy is transferred as heat between them.
\( \mathrm{q_{lost} = -q_{gained}} \)
![]()
At equilibrium:
- The total energy of the combined system remains constant (law of conservation of energy).
- Energy continues to be exchanged at the microscopic level, but there is no net transfer of heat.
- The temperatures of both substances become equal, implying their average kinetic energies are identical.
Thermal equilibrium occurs when two systems in contact reach the same temperature. No net heat flows between them because their particles have the same average kinetic energy.
| Concept | Condition | Energy Transfer | Particle Behavior |
|---|---|---|---|
| Before Equilibrium | \( \mathrm{T_{hot} > T_{cold}} \) | Energy flows from hot → cold | Faster particles transfer energy to slower ones |
| At Equilibrium | \( \mathrm{T_{hot} = T_{cold}} \) | No net energy transfer | Average kinetic energy of both is equal |
| Energy Conservation | Always true | \( \mathrm{q_{lost} = -q_{gained}} \) | Total system energy remains constant |
Example :
A 250 g piece of aluminum at 90 °C is placed in 150 g of water at 25 °C inside an insulated cup. The final temperature of the system is 33 °C. Explain what happens at the particle level and verify energy conservation.
▶️ Answer / Explanation
Step 1: Calculate the energy lost by aluminum and gained by water.
For aluminum: \( \mathrm{c_{Al} = 0.897\ J\ g^{-1}\ ^\circ C^{-1}} \)
\( \mathrm{q_{Al} = (250)(0.897)(33 – 90) = -12797\ J} \)
For water: \( \mathrm{c_{H_2O} = 4.18\ J\ g^{-1}\ ^\circ C^{-1}} \)
\( \mathrm{q_{H_2O} = (150)(4.18)(33 – 25) = +12540\ J} \)
Step 2: The total energy change ≈ 0 (small rounding difference): \( \mathrm{q_{Al} + q_{H_2O} \approx 0} \)
Step 3: The final temperature (33 °C) is the same for both — meaning no net heat flow remains.
Step 4: Microscopically: Fast-moving Al atoms transferred kinetic energy to slower-moving water molecules until both reached the same average kinetic energy.
Final Answer: The system has reached thermal equilibrium; energy lost by Al = energy gained by water, and both have the same temperature (33 °C).
Example :
Describe what happens when a hot cup of coffee is left on a table in a room at 22 °C.
▶️ Answer / Explanation
Step 1: The coffee’s molecules initially move faster (higher kinetic energy) than the air molecules around it.
Step 2: Energy transfers from coffee → surrounding air via molecular collisions and convection.
Step 3: As heat is lost, coffee cools while air slightly warms — until their temperatures equalize.
Step 4: At equilibrium, coffee and air have the same average molecular kinetic energy, and no further net heat transfer occurs.
Final Answer: The coffee cools and the air warms until both reach the same temperature — the system is in thermal equilibrium, with identical average kinetic energies.
