AP Chemistry 6.4 Heat Capacity and Calorimetry Study Notes - New Syllabus Effective fall 2024
AP Chemistry 6.4 Heat Capacity and Calorimetry Study Notes.- New syllabus
AP Chemistry 6.4 Heat Capacity and Calorimetry Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Calculate the heat q absorbed or released by a system undergoing heating/cooling based on the amount of the substance, the heat capacity, and the change in temperature.
Key Concepts:
- Energy Transfers
- Calorimetry Calculations
Heat Transfer and the Equation \( \mathrm{q = mc\Delta T} \)
The amount of heat energy gained or lost by a substance depends on its mass, specific heat capacity, and temperature change.
When a cooler object and a warmer object come into contact, thermal energy is transferred from the warmer body to the cooler one. This process continues until both reach the same temperature thermal equilibrium. The quantity of energy transferred as heat can be calculated using the heat transfer equation: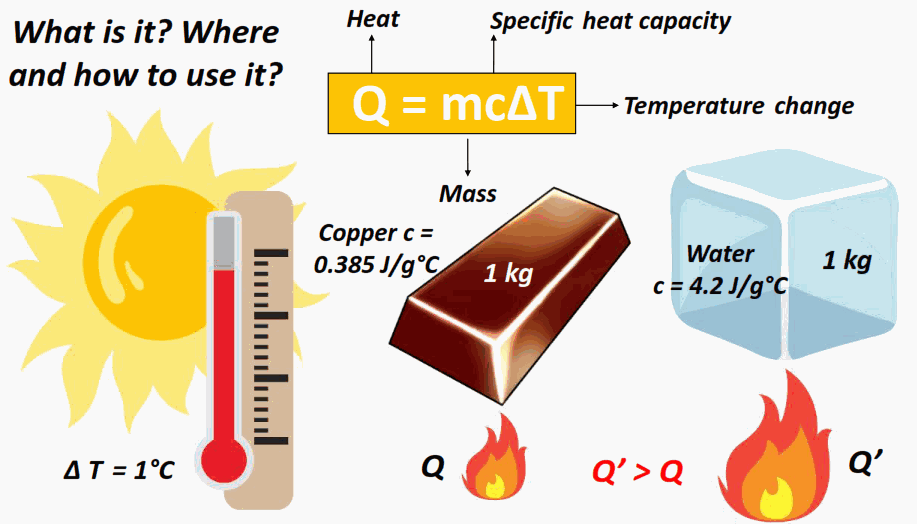
\( \mathrm{q = mc\Delta T} \)
Where:
- \( \mathrm{q} \): heat energy absorbed or released (J)
- \( \mathrm{m} \): mass of the substance (g)
- \( \mathrm{c} \): specific heat capacity (\( \mathrm{J\,g^{-1}\,K^{-1}} \))
- \( \mathrm{\Delta T} \): temperature change (\( \mathrm{T_{final} – T_{initial}} \)) (K or °C)
Key Properties:
- The direction of heat transfer is always from the warmer to the cooler object.
- If heat is absorbed by the system, \( \mathrm{q > 0} \) (endothermic).
- If heat is released by the system, \( \mathrm{q < 0} \) (exothermic).
- The specific heat capacity (\( \mathrm{c} \)) determines how much energy is needed to change temperature by 1 K per gram of substance.
- Calorimetry experiments use this relationship to measure energy transfer between substances.
Key Idea: Heat transfer between substances can be quantified using \( \mathrm{q = mc\Delta T} \), which relates the energy absorbed or released to mass, heat capacity, and temperature change.
| Quantity | Symbol | Unit | Meaning |
|---|---|---|---|
| Heat energy | \( \mathrm{q} \) | Joules (J) | Energy transferred as heat |
| Specific heat capacity | \( \mathrm{c} \) | \( \mathrm{J\,g^{-1}\,K^{-1}} \) | Energy required to raise 1 g of substance by 1 K |
| Temperature change | \( \mathrm{\Delta T} \) | K or °C | Difference between final and initial temperature |
Example:
A 50.0 g sample of water is heated from 20.0 °C to 65.0 °C. Calculate the heat absorbed by the water. (\( \mathrm{c_{water} = 4.18\ J\,g^{-1}\,°C^{-1}} \))
▶️ Answer/Explanation
Step 1: Write the equation \( \mathrm{q = mc\Delta T} \).
Step 2: Calculate \( \mathrm{\Delta T = T_f – T_i = 65.0 – 20.0 = 45.0\ °C} \).
Step 3: Substitute values: \( \mathrm{q = (50.0\ g)(4.18\ J\,g^{-1}\,°C^{-1})(45.0\ °C)} \)
Step 4: \( \mathrm{q = 9410\ J} \)
Final Answer: The water absorbs \( \mathrm{9.41 \times 10^3\ J} \) (9.41 kJ) of heat.
The First Law of Thermodynamics (Energy Conservation)
The first law of thermodynamics states that energy cannot be created or destroyed, only transformed from one form to another or transferred between systems and surroundings. The total energy of the universe remains constant. This principle underlies all thermochemical and calorimetric analyses.
Mathematical Representation:
\( \mathrm{\Delta U = q + w} \)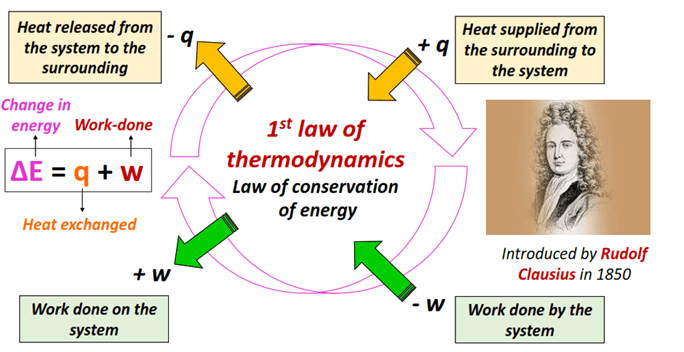
Where:
- \( \mathrm{\Delta U} \): change in internal energy of the system
- \( \mathrm{q} \): heat added to the system (J)
- \( \mathrm{w} \): work done on or by the system (J)
Sign Conventions:
- \( \mathrm{q > 0} \): heat absorbed by the system (endothermic)
- \( \mathrm{q < 0} \): heat released by the system (exothermic)
- \( \mathrm{w > 0} \): work done on the system
- \( \mathrm{w < 0} \): work done by the system
Key Properties:
- Energy changes in a system result from both heat transfer and work.
- In chemical reactions, the change in internal energy can be measured by calorimetry under constant volume or constant pressure conditions.
- At constant pressure, the heat flow corresponds to the change in enthalpy (\( \mathrm{\Delta H} \)).
The total energy of a system and its surroundings is conserved. Energy lost by one is gained by the other, consistent with \( \mathrm{\Delta U = q + w} \).
| Type of Process | Condition | Energy Expression | Interpretation |
|---|---|---|---|
| Constant Volume | No work done (\( \mathrm{w = 0} \)) | \( \mathrm{\Delta U = q_v} \) | Energy change equals heat at constant volume |
| Constant Pressure | System can expand/contract | \( \mathrm{\Delta H = q_p} \) | Heat equals enthalpy change of the system |
Example :
A gas absorbs \( \mathrm{325\ J} \) of heat while expanding and doing \( \mathrm{105\ J} \) of work on the surroundings. Calculate the change in internal energy of the system. Then determine whether the process is endothermic or exothermic.
▶️ Answer/Explanation
Step 1: Write the first law of thermodynamics: \( \mathrm{\Delta U = q + w} \).
Step 2: Substitute known values. The system absorbs heat: \( \mathrm{q = +325\ J} \). The system does work on surroundings: \( \mathrm{w = -105\ J} \).
Step 3: Calculate: \( \mathrm{\Delta U = (+325) + (-105) = +220\ J} \).
Step 4: Interpretation: Since \( \mathrm{\Delta U > 0} \), the internal energy of the system increases — the process is endothermic.
Final Answer: \( \mathrm{\Delta U = +220\ J} \), endothermic process.
Specific Heat Capacity Differences
When equal quantities of thermal energy are transferred to different substances, they do not experience the same temperature change. This is because each substance has a unique property called its specific heat capacity, \( \mathrm{c} \), which measures how much energy is required to raise the temperature of 1 gram of that substance by 1 kelvin (or 1 °C).
Mathematical Relation: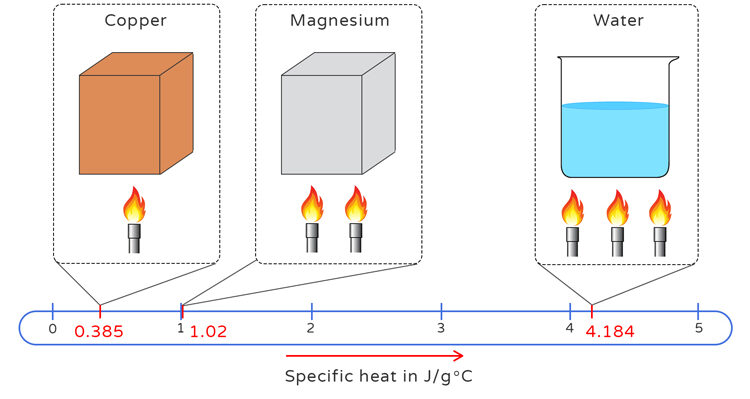
\( \mathrm{q = mc\Delta T} \)
Key Relationships:
- For a given amount of heat \( \mathrm{q} \): \( \mathrm{\Delta T \propto \dfrac{1}{c}} \). (The greater the specific heat, the smaller the temperature change for the same energy input.)
- Metals typically have low \( \mathrm{c} \) values, so they heat up and cool down quickly.
- Water has a high \( \mathrm{c} \) value (4.18 \( \mathrm{J\,g^{-1}\,°C^{-1}} \)), so it resists temperature changes — a key factor in climate moderation and biological stability.
Key Idea: Substances with higher specific heat capacities undergo smaller temperature changes for the same energy input, while those with lower specific heat capacities undergo larger temperature changes.
| Substance | Specific Heat Capacity (\( \mathrm{J\,g^{-1}\,°C^{-1}} \)) | Relative Response to Heating |
|---|---|---|
| Water | 4.18 | Heats up slowly; stores heat efficiently |
| Aluminum | 0.90 | Heats up faster than water |
| Iron | 0.45 | Heats up very quickly; poor heat storage |
Example:
Equal masses (100 g each) of liquid water and aluminum metal are both heated using 418 J of energy. Calculate the temperature increase for each and determine which material experiences the larger temperature change.
▶️ Answer/Explanation
Step 1: Write the heat equation \( \mathrm{q = mc\Delta T} \). Rearrange for \( \mathrm{\Delta T} \): \( \mathrm{\Delta T = \dfrac{q}{mc}} \).
Step 2: For water: \( \mathrm{q = 418\ J} \), \( \mathrm{m = 100\ g} \), \( \mathrm{c = 4.18\ J\,g^{-1}\,°C^{-1}} \)
\( \mathrm{\Delta T_{H_2O} = \dfrac{418}{(100)(4.18)} = 1.00\ °C} \)
Step 3: For aluminum: \( \mathrm{q = 418\ J} \), \( \mathrm{m = 100\ g} \), \( \mathrm{c = 0.90\ J\,g^{-1}\,°C^{-1}} \)
\( \mathrm{\Delta T_{Al} = \dfrac{418}{(100)(0.90)} = 4.64\ °C} \)
Step 4: Compare results: The aluminum sample undergoes a larger temperature rise (4.64 °C vs. 1.00 °C).
Step 5: Interpretation: The smaller the specific heat capacity, the greater the temperature change for the same energy input.
Final Answer: \( \mathrm{\Delta T_{H_2O} = 1.00\ °C} \); \( \mathrm{\Delta T_{Al} = 4.64\ °C} \). Aluminum heats up more rapidly because of its lower specific heat capacity.
Heating and Cooling of Systems
When a system is heated, its internal energy increases as the kinetic energy of its particles rises. Conversely, when a system cools, its internal energy decreases as particles slow down. This change in energy can occur due to heat transfer or work, but in most simple heating and cooling cases, the energy change is due to heat flow described by \( \mathrm{q = mc\Delta T} \).
Key Concept :
- Heating a system increases its internal energy — the temperature rises.
- Cooling a system decreases its internal energy — the temperature falls.
- The rate of temperature change depends on the specific heat capacity of the substance and the amount of heat transferred.
- On a heating/cooling curve, sloped regions represent temperature change (\( \mathrm{q = mc\Delta T} \)), while flat regions represent phase changes (\( \mathrm{q = mL} \)).
Heating increases the average kinetic energy of particles, while cooling decreases it. The quantitative relationship between heat flow and temperature change is governed by \( \mathrm{q = mc\Delta T} \).
Heating and Cooling Curve Explanation:
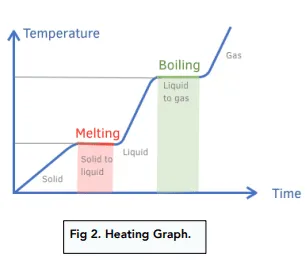
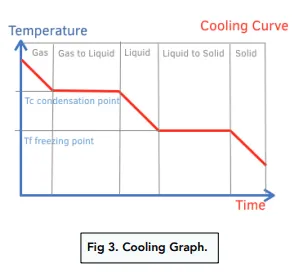
| Process | Equation | Temperature Change? |
|---|---|---|
| Solid warming | \( \mathrm{q = mc_{solid}\Delta T} \) | Yes |
| Melting (fusion) | \( \mathrm{q = mL_f} \) | No |
| Liquid warming | \( \mathrm{q = mc_{liquid}\Delta T} \) | Yes |
| Boiling (vaporization) | \( \mathrm{q = mL_v} \) | No |
| Gas heating | \( \mathrm{q = mc_{gas}\Delta T} \) | Yes |
Note: Cooling follows the same curve in reverse — energy is released as temperature or phase decreases.
Example :
How much heat (in kJ) is required to convert 50.0 g of ice at −10.0 °C to steam at 120.0 °C? Use the following data:
- \( \mathrm{c_{ice} = 2.09\ J\,g^{-1}\,°C^{-1}} \)
- \( \mathrm{c_{water} = 4.18\ J\,g^{-1}\,°C^{-1}} \)
- \( \mathrm{c_{steam} = 2.00\ J\,g^{-1}\,°C^{-1}} \)
- \( \mathrm{L_f = 334\ J\,g^{-1}} \)
- \( \mathrm{L_v = 2260\ J\,g^{-1}} \)
▶️ Answer/Explanation
Step 1: Break the process into stages:
- Heating ice from −10 °C to 0 °C
- Melting ice at 0 °C
- Heating water from 0 °C to 100 °C
- Vaporizing water at 100 °C
- Heating steam from 100 °C to 120 °C
Step 2: Calculate energy for each stage.
1️ \( \mathrm{q_1 = (50.0)(2.09)(10.0) = 1045\ J} \)
2️ \( \mathrm{q_2 = (50.0)(334) = 16,700\ J} \)
3️ \( \mathrm{q_3 = (50.0)(4.18)(100.0) = 20,900\ J} \)
4️ \( \mathrm{q_4 = (50.0)(2260) = 113,000\ J} \)
5️ \( \mathrm{q_5 = (50.0)(2.00)(20.0) = 2000\ J} \)
Step 3: Add total heat:
\( \mathrm{q_{total} = 1045 + 16,700 + 20,900 + 113,000 + 2000 = 153,645\ J} \)
Step 4: Convert to kJ: \( \mathrm{q_{total} = 153.6\ kJ} \)
Final Answer: \( \mathrm{153.6\ kJ} \) of heat is required to convert 50.0 g of ice at −10 °C to steam at 120 °C.
Specific and Molar Heat Capacities
The amount of heat required to change the temperature of a substance depends not only on its mass but also on whether the heat capacity is expressed per gram or per mole. These two related quantities — specific heat capacity and molar heat capacity — describe how substances respond to thermal energy input.
Definitions:
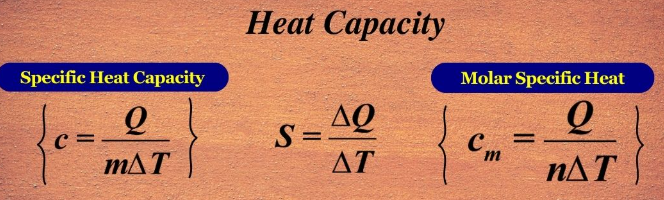
- Specific Heat Capacity (\( \mathrm{c} \)): The heat required to raise the temperature of 1 gram of a substance by 1 K (or 1 °C). \( \mathrm{q = mc\Delta T} \)
- Molar Heat Capacity (\( \mathrm{C_m} \)): The heat required to raise the temperature of 1 mole of a substance by 1 K (or 1 °C). \( \mathrm{q = nC_m\Delta T} \)
Relationship Between Them:
\( \mathrm{C_m = M \times c} \)
where \( \mathrm{M} \) = molar mass of the substance.
Specific heat capacity measures heat per unit mass, while molar heat capacity measures heat per mole. Both describe the thermal “resistance” of a substance to temperature change.
| Quantity | Symbol | Units | Description |
|---|---|---|---|
| Specific Heat Capacity | \( \mathrm{c} \) | \( \mathrm{J\,g^{-1}\,K^{-1}} \) | Heat required per gram per degree |
| Molar Heat Capacity | \( \mathrm{C_m} \) | \( \mathrm{J\,mol^{-1}\,K^{-1}} \) | Heat required per mole per degree |
Typical Values (at 25 °C):
| Substance | \( \mathrm{c\ (J\,g^{-1}\,K^{-1})} \) | \( \mathrm{C_m\ (J\,mol^{-1}\,K^{-1})} \) |
|---|---|---|
| Water (\( \mathrm{H_2O(l)} \)) | 4.18 | 75.4 |
| Copper (\( \mathrm{Cu(s)} \)) | 0.385 | 24.4 |
| Ethanol (\( \mathrm{C_2H_5OH(l)} \)) | 2.46 | 113 |
Example :
A 25.0 g sample of copper metal at 150.0 °C is placed into 100.0 g of water at 25.0 °C in an insulated container. Assuming no heat is lost to the surroundings, calculate the final equilibrium temperature of the mixture. (Given: \( \mathrm{c_{Cu} = 0.385\ J\,g^{-1}\,°C^{-1}} \), \( \mathrm{c_{H_2O} = 4.18\ J\,g^{-1}\,°C^{-1}} \))
▶️ Answer/Explanation
Step 1: Heat lost by copper = heat gained by water. \( \mathrm{q_{Cu} = -q_{H_2O}} \)
Step 2: Use \( \mathrm{q = mc\Delta T} \) for both substances.
For copper: \( \mathrm{q_{Cu} = (25.0)(0.385)(T_f – 150.0)} \)
For water: \( \mathrm{q_{H_2O} = (100.0)(4.18)(T_f – 25.0)} \)
Step 3: Set \( \mathrm{q_{Cu} + q_{H_2O} = 0} \):
\( \mathrm{(25.0)(0.385)(T_f – 150.0) + (100.0)(4.18)(T_f – 25.0) = 0} \)
Step 4: Simplify:
\( \mathrm{9.625T_f – 1443.8 + 418T_f – 104500 = 0} \)
\( \mathrm{427.6T_f = 105943.8} \)
\( \mathrm{T_f = 247.8\ °C} \) — wait, this seems too high. Let’s check sign direction.
Correct form (since copper cools, water warms):
\( \mathrm{(25.0)(0.385)(150.0 – T_f) = (100.0)(4.18)(T_f – 25.0)} \)
\( \mathrm{9.625(150.0 – T_f) = 418(T_f – 25.0)} \)
\( \mathrm{1443.8 – 9.625T_f = 418T_f – 10450} \)
\( \mathrm{11893.8 = 427.6T_f} \)
\( \mathrm{T_f = 27.8\ °C} \)
Step 5: Final Answer: The equilibrium temperature of the mixture is \( \mathrm{27.8\ °C} \).
The large heat capacity of water causes only a small temperature increase.
Energy Changes in Chemical Systems
In a chemical or physical process, the energy of a system can increase or decrease depending on whether the system absorbs or releases energy. These energy changes are governed by the first law of thermodynamics and expressed through heat (\( \mathrm{q} \)) and work (\( \mathrm{w} \)). In chemical reactions, most of the energy change occurs as heat flow, measurable as an enthalpy change \( \mathrm{(\Delta H)} \).
The enthalpy change of a system, \( \mathrm{\Delta H} \), represents the heat absorbed or released at constant pressure: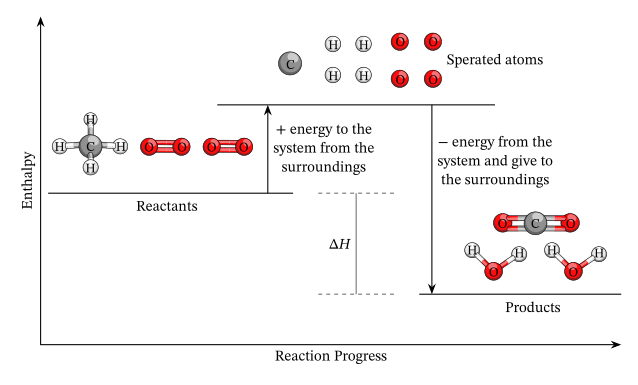
\( \mathrm{\Delta H = H_{products} – H_{reactants}} \)
Key Relationships:
- \( \mathrm{\Delta H > 0} \): Endothermic — energy absorbed by the system from surroundings.
- \( \mathrm{\Delta H < 0} \): Exothermic — energy released by the system to surroundings.
- At constant pressure: \( \mathrm{q_p = \Delta H} \).
- Energy is conserved: energy lost by surroundings equals energy gained by the system (and vice versa).
Energy changes in a chemical system result from the breaking and forming of chemical bonds. Breaking bonds requires energy (endothermic), while forming bonds releases energy (exothermic). The overall \( \mathrm{\Delta H} \) depends on the balance of these two processes.
| Type of Process | Sign of \( \mathrm{\Delta H} \) | Energy Flow | Effect on System |
|---|---|---|---|
| Endothermic | \( \mathrm{+} \) | Energy absorbed from surroundings | Temperature of surroundings decreases |
| Exothermic | \( \mathrm{−} \) | Energy released to surroundings | Temperature of surroundings increases |
Note: Calorimetry experiments often determine \( \mathrm{\Delta H} \) indirectly by measuring the temperature change of the surroundings and applying \( \mathrm{q = -m_{surr}c\Delta T} \).
Example :
A 0.100 mol sample of hydrogen gas reacts completely with oxygen to form liquid water at 25 °C. Calculate the heat released by this reaction under constant pressure. (Given: \( \mathrm{2H_2(g) + O_2(g) \rightarrow 2H_2O(l)} \), \( \mathrm{\Delta H = -571.6\ kJ/mol\ H_2O(l)} \))
▶️ Answer/Explanation
Step 1: Identify given data and reaction stoichiometry.
From the reaction: 2 mol \( \mathrm{H_2} \) produce 2 mol \( \mathrm{H_2O(l)} \). Thus, 1 mol \( \mathrm{H_2} \) produces 1 mol \( \mathrm{H_2O(l)} \) with \( \mathrm{\Delta H = -571.6\ kJ} \).
Step 2: For 0.100 mol \( \mathrm{H_2} \):
\( \mathrm{q = n\Delta H = (0.100\ mol)(-571.6\ kJ/mol)} \)
\( \mathrm{q = -57.16\ kJ} \)
Step 3: Interpretation:
The negative sign indicates that the system releases energy to the surroundings — the reaction is exothermic.
Step 4: If this reaction occurred in a calorimeter, the surroundings (water) would absorb 57.16 kJ of heat, leading to a measurable temperature rise.
Final Answer: \( \mathrm{q = -57.16\ kJ} \) (exothermic).
Calorimetry and Dissolution Processes
Calorimetry is the experimental method used to measure heat flow during chemical and physical processes. When a solute dissolves in a solvent, heat is either absorbed or released depending on whether the process is endothermic or exothermic. The temperature change of the surroundings (usually water) reveals the energy change of the dissolution process.
Key Principle: In calorimetry, the law of conservation of energy applies: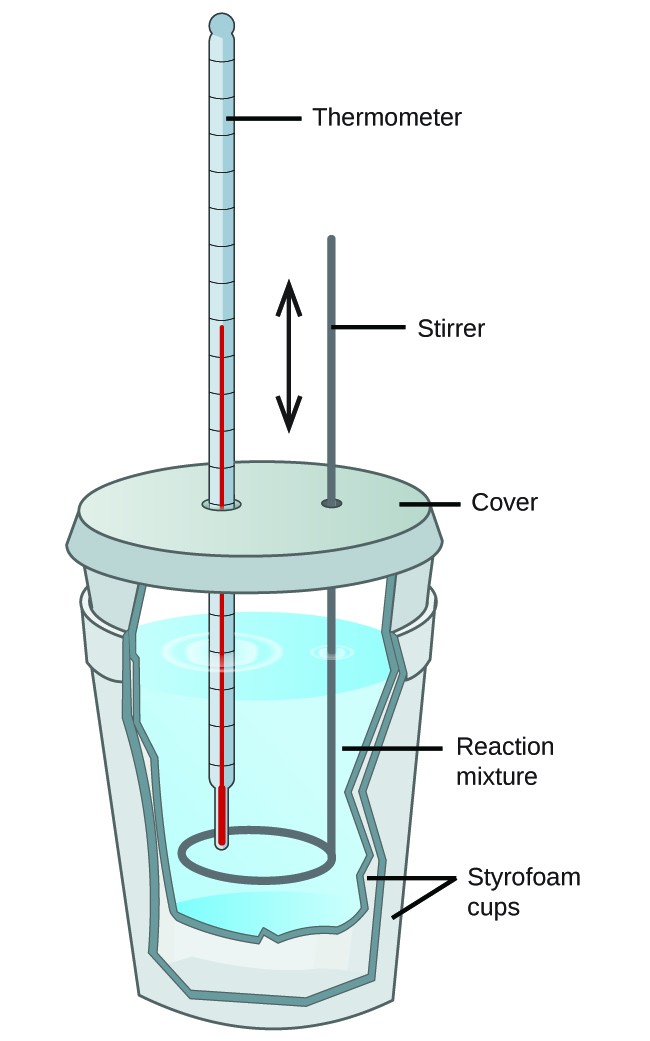
\( \mathrm{q_{system} = -q_{surroundings}} \)
In a dissolution process:
- If the solution absorbs heat from water, it is endothermic (temperature of water decreases).
- If the solution releases heat to water, it is exothermic (temperature of water increases).
Formula for Calculation:
\( \mathrm{q_{solution} = m_{water}c_{water}\Delta T} \)
and since \( \mathrm{q_{system} = -q_{solution}} \):
\( \mathrm{q_{dissolution} = -m_{water}c_{water}\Delta T} \)
The direction and magnitude of temperature change during dissolution determine whether the process is endothermic or exothermic, and allow the enthalpy change per mole (\( \mathrm{\Delta H_{soln}} \)) to be calculated.
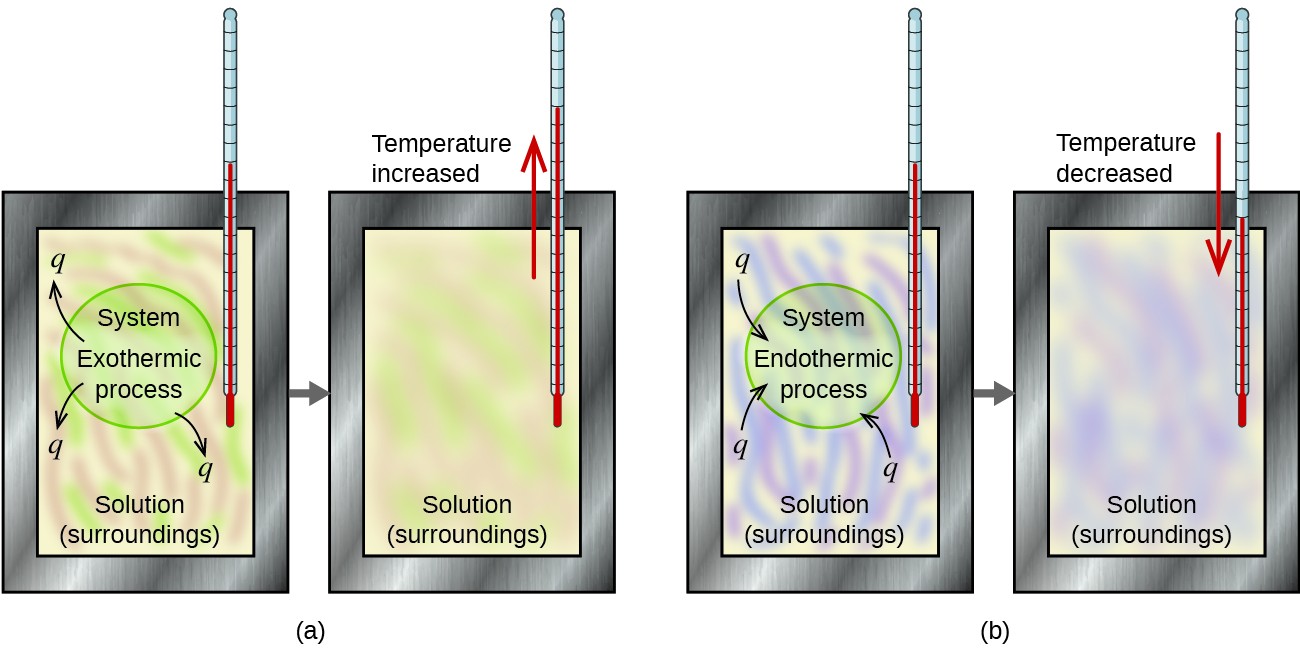
| Process Type | Temperature Change | Sign of \( \mathrm{\Delta H_{soln}} \) | Example |
|---|---|---|---|
| Exothermic Dissolution | Temperature of water increases | Negative | NaOH(s) dissolving in water |
| Endothermic Dissolution | Temperature of water decreases | Positive | NH₄NO₃(s) dissolving in water (cold packs) |
Example:
When 5.00 g of solid ammonium nitrate (\( \mathrm{NH_4NO_3} \)) dissolves in 50.0 g of water, the temperature of the solution decreases from 25.0 °C to 20.3 °C. Calculate the molar enthalpy of solution (\( \mathrm{\Delta H_{soln}} \)) in \( \mathrm{kJ/mol} \). (Assume the specific heat capacity of the solution = \( \mathrm{4.18\ J\,g^{-1}\,°C^{-1}} \), and total mass of solution = 55.0 g.)
▶️ Answer/Explanation
Step 1: Determine the heat change in the solution (surroundings):
\( \mathrm{q_{solution} = m\,c\,\Delta T} \)
\( \mathrm{q_{solution} = (55.0\ g)(4.18\ J\,g^{-1}\,°C^{-1})(20.3 – 25.0)} \)
\( \mathrm{q_{solution} = (55.0)(4.18)(-4.7) = -1081.9\ J} \)
Step 2: The system (the dissolution) absorbs this energy:
\( \mathrm{q_{dissolution} = -q_{solution} = +1081.9\ J} \)
Step 3: Convert to kJ: \( \mathrm{q_{dissolution} = 1.082\ kJ} \)
Step 4: Determine moles of \( \mathrm{NH_4NO_3} \): \( \mathrm{M = 80.04\ g/mol} \) \( \mathrm{n = \dfrac{5.00}{80.04} = 0.0625\ mol} \)
Step 5: Calculate molar enthalpy of solution:
\( \mathrm{\Delta H_{soln} = \dfrac{q_{dissolution}}{n} = \dfrac{1.082}{0.0625} = 17.3\ kJ/mol} \)
Step 6: Interpretation:
The positive sign indicates that the process is endothermic — the dissolution of \( \mathrm{NH_4NO_3} \) absorbs heat, which causes the solution’s temperature to decrease.
Final Answer: \( \mathrm{\Delta H_{soln} = +17.3\ kJ/mol} \) (endothermic).
