AP Chemistry 6.5 Energy of Phase Changes Study Notes - New Syllabus Effective fall 2024
AP Chemistry 6.5 Energy of Phase Changes Study Notes.- New syllabus
AP Chemistry 6.5 Energy of Phase Changes Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain changes in the heat q absorbed or released by a system undergoing a phase transition based on the amount of the substance in moles and the molar enthalpy of the phase transition.
Key Concepts:
- Energy Transfer During Phase Changes
- Complementary Energies for Phase Changes
Energy Transfer During Phase Changes
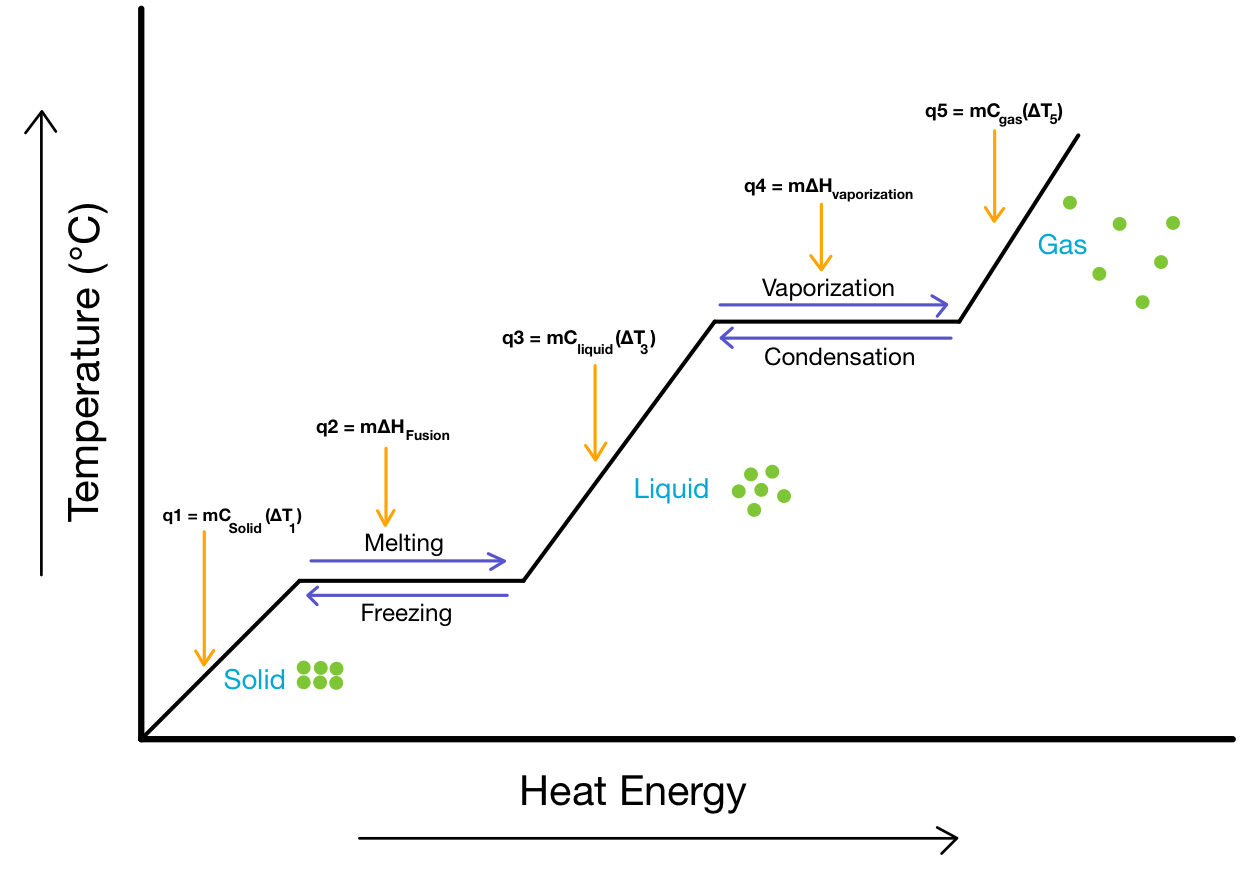
During a phase change, energy is either absorbed or released by a system without changing its temperature.
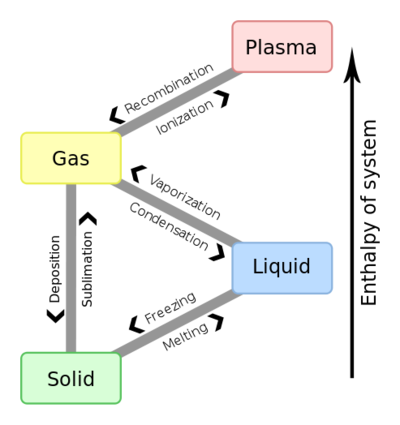
- When a substance melts or boils, energy is absorbed → particles move farther apart.
- When a substance freezes or condenses, energy is released → particles move closer together.
- Temperature remains constant during the phase change because energy changes potential energy, not kinetic energy.
Key Formulas
1. General Formula: \( \mathrm{q = n \Delta H} \)
where:
- \( \mathrm{q} \): energy transferred (J or kJ)
- \( \mathrm{n} \): moles of substance
- \( \mathrm{\Delta H} \): molar enthalpy of phase change (kJ/mol)
2. For melting/freezing: \( \mathrm{q = n \Delta H_{fus}} \)
3. For vaporization/condensation: \( \mathrm{q = n \Delta H_{vap}} \)
Energy Direction and System Change
| Process | Direction of Energy Flow | Type of Process | Effect on System |
|---|---|---|---|
| Melting (solid → liquid) | Energy absorbed | Endothermic | System energy increases |
| Boiling (liquid → gas) | Energy absorbed | Endothermic | System energy increases |
| Freezing (liquid → solid) | Energy released | Exothermic | System energy decreases |
| Condensation (gas → liquid) | Energy released | Exothermic | System energy decreases |
Important Points
- The temperature remains constant during melting, boiling, freezing, or condensation.
- Energy absorbed or released equals the enthalpy of phase change.
- After the phase change, further heating or cooling changes the kinetic energy (temperature).
Example:
Calculate the energy required to melt 50.0 g of ice at 0 °C.
(Given: \( \mathrm{\Delta H_{fus}(H_2O) = 6.01\ kJ/mol} \))
▶️ Answer / Explanation
Step 1: Calculate moles of ice: \( \mathrm{n = \dfrac{50.0}{18.02} = 2.77\ mol} \)
Step 2: Use \( \mathrm{q = n \Delta H_{fus}} \): \( \mathrm{q = (2.77)(6.01) = 16.7\ kJ} \)
Step 3: Interpretation:
- Energy is absorbed by the system → endothermic.
- Temperature remains constant at 0 °C until all ice melts.
Final Answer: \( \mathrm{q = +16.7\ kJ} \) of energy absorbed to melt 50.0 g of ice.
Complementary Energies for Phase Changes
The energy absorbed during a phase change in one direction is equal in magnitude and opposite in sign to the energy released during the reverse (complementary) phase change.
- For vaporization and condensation: \( \mathrm{\Delta H_{cond} = -\Delta H_{vap}} \)
- For fusion and freezing: \( \mathrm{\Delta H_{freeze} = -\Delta H_{fus}} \)
Key Formulas / Sign Convention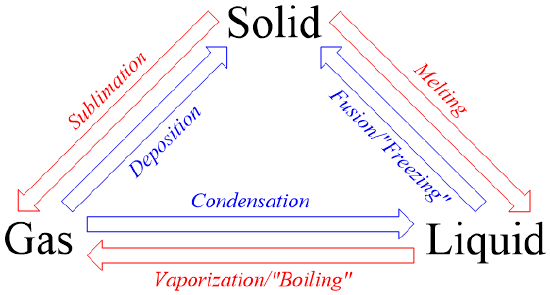
1. General Relation: \( \mathrm{q = n \Delta H} \)
2. Complementary Phase Change Relations:
\( \mathrm{\Delta H_{cond} = -\Delta H_{vap}} \)
\( \mathrm{\Delta H_{freeze} = -\Delta H_{fus}} \)
3. Sign Convention: \( \mathrm{\Delta H > 0} \) → endothermic (energy absorbed) \( \mathrm{\Delta H < 0} \) → exothermic (energy released)
Important Points
- The magnitudes of enthalpy changes for complementary phase transitions are identical; only the signs differ.
- Endothermic → system absorbs energy (melting, vaporization).
- Exothermic → system releases energy (freezing, condensation).
- These relationships allow energy calculations for reverse processes without needing new experimental data.
Example :
Given \( \mathrm{\Delta H_{vap}(H_2O) = +40.65\ kJ\ mol^{-1}} \), calculate the energy change when 0.250 mol of water vapor condenses at 100 °C.
▶️ Answer / Explanation
Step 1: Use the complementary relation: \( \mathrm{\Delta H_{cond} = -\Delta H_{vap} = -40.65\ kJ\ mol^{-1}} \).
Step 2: \( \mathrm{q = n \Delta H_{cond} = (0.250)(-40.65) = -10.16\ kJ} \).
Step 3: The negative sign indicates energy is released to the surroundings.
Final Answer: \( \mathrm{q = -10.16\ kJ} \) → 10.16 kJ of energy is released when 0.250 mol of steam condenses.
Example :
The enthalpy of fusion for water is \( \mathrm{\Delta H_{fus} = +6.01\ kJ\ mol^{-1}} \). Calculate the energy change when 36.0 g of liquid water freezes at 0 °C.
▶️ Answer / Explanation
Step 1: Determine moles of water: \( \mathrm{n = \dfrac{36.0}{18.02} = 2.00\ mol} \).
Step 2: Freezing is the reverse of fusion: \( \mathrm{\Delta H_{freeze} = -\Delta H_{fus} = -6.01\ kJ\ mol^{-1}} \).
Step 3: \( \mathrm{q = n \Delta H_{freeze} = (2.00)(-6.01) = -12.0\ kJ} \).
Step 4: The negative value shows energy is released during freezing.
Final Answer: \( \mathrm{q = -12.0\ kJ} \) → 12.0 kJ of energy released as 36.0 g of water freezes.
