AP Chemistry 6.6 Introduction to Enthalpy of Reaction Study Notes - New Syllabus Effective fall 2024
AP Chemistry 6.6 Introduction to Enthalpy of Reaction Study Notes.- New syllabus
AP Chemistry 6.6 Introduction to Enthalpy of Reaction Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain changes in the heat q absorbed or released by a system undergoing a phase transition based on the amount of the substance in moles and the molar enthalpy of the phase transition.
Key Concepts:
- Enthalpy Change and Heat Energy at Constant Pressure
- Energy Exchange with Surroundings During Chemical Reactions
- Chemical Potential Energy, Bond Changes, and Temperature Effects
Enthalpy Change and Heat Energy at Constant Pressure
The enthalpy change (\( \mathrm{\Delta H} \)) of a chemical reaction represents the amount of heat energy absorbed or released by the system when the reaction occurs at constant pressure.
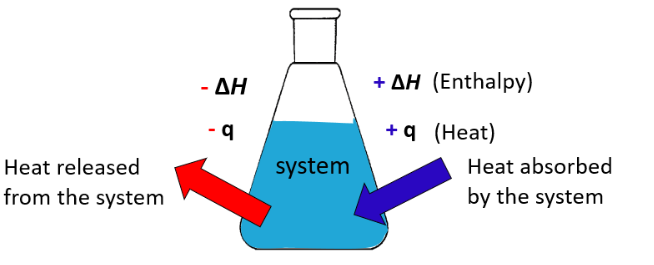
- \( \mathrm{\Delta H > 0} \): Reaction absorbs heat → endothermic.
- \( \mathrm{\Delta H < 0} \): Reaction releases heat → exothermic.
Definition:
The enthalpy change of a reaction is equal to the heat exchanged with the surroundings at constant pressure:
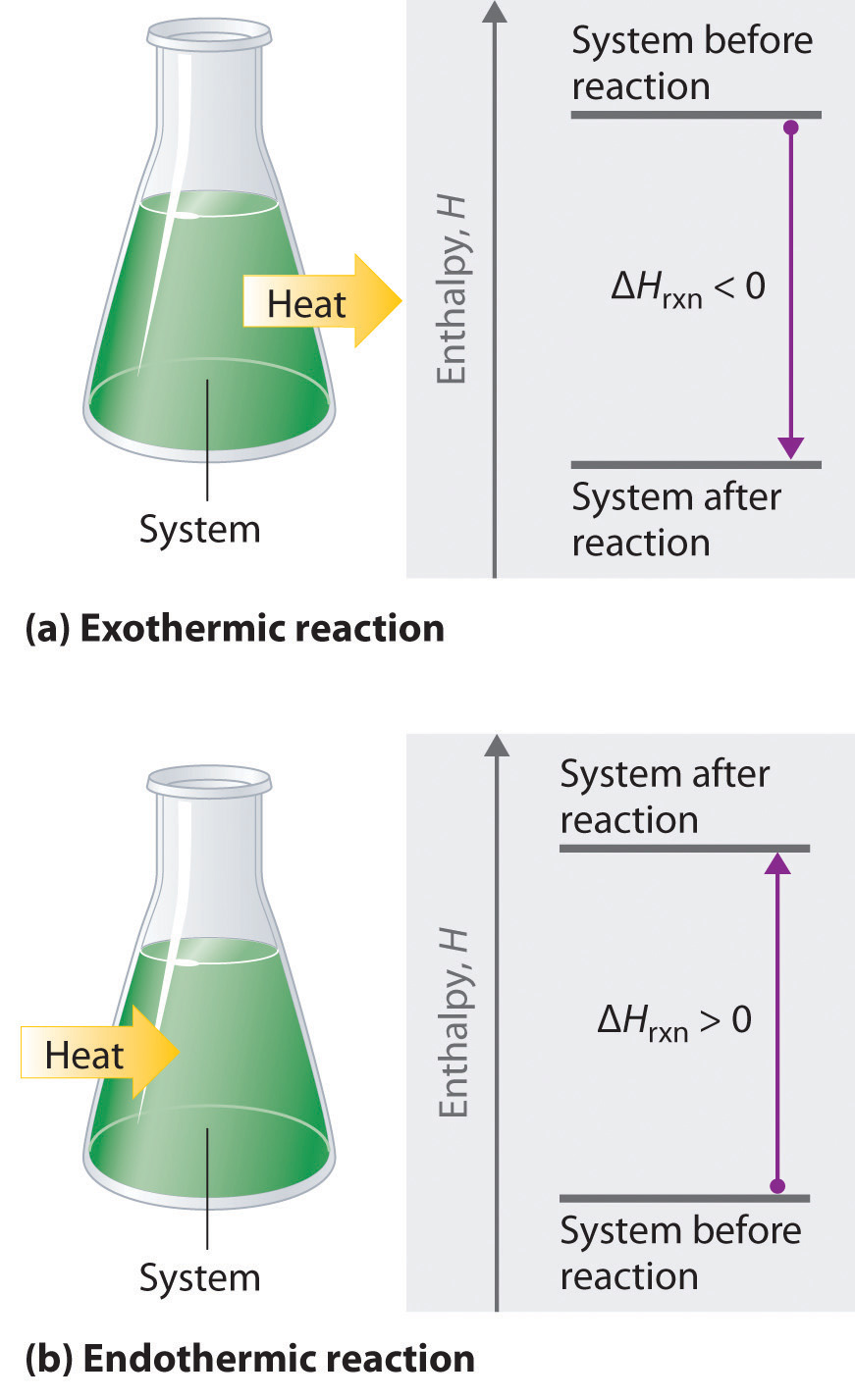
\( \mathrm{\Delta H = q_p} \)
- \( \mathrm{q_p} \): heat exchanged at constant pressure (J or kJ)
- \( \mathrm{\Delta H} \): enthalpy change of the reaction (kJ mol⁻¹)
Key Relationships
- \( \mathrm{\Delta H} \) depends on the difference between the total energy of bonds broken and bonds formed.
- At constant pressure, enthalpy change = heat energy measured by a calorimeter.
- Energy conservation: \( \mathrm{q_{system} = -q_{surroundings}} \).
Important Notes
- Exothermic: Energy released → temperature of surroundings increases.
- Endothermic: Energy absorbed → temperature of surroundings decreases.
- The unit of enthalpy change is typically \( \mathrm{kJ\ mol^{-1}} \).
Example:
The combustion of methane can be represented as:
\( \mathrm{CH_4(g) + 2O_2(g) \rightarrow CO_2(g) + 2H_2O(g)} \)
The enthalpy change for this reaction is \( \mathrm{\Delta H = -890\ kJ\ mol^{-1}} \).
▶️ Answer / Explanation
Step 1: The negative sign indicates energy release → exothermic reaction.
Step 2: This means 890 kJ of heat is released for every 1 mol of methane burned at constant pressure.
Step 3: In a calorimeter, the surroundings (air, water) would gain this energy, increasing their temperature.
Final Answer: \( \mathrm{\Delta H = -890\ kJ\ mol^{-1}} \); the system loses energy, surroundings gain it as heat.
Energy Exchange with Surroundings During Chemical Reactions
When a chemical reaction occurs, the products may initially be at a different temperature than their surroundings. To reach thermal equilibrium, energy is exchanged between the system (reactants/products) and the surroundings.
At constant pressure, heat transfer between a system and its surroundings results from the conversion of chemical potential energy into thermal energy or vice versa.
\( \mathrm{q_{system} = -q_{surroundings}} \)
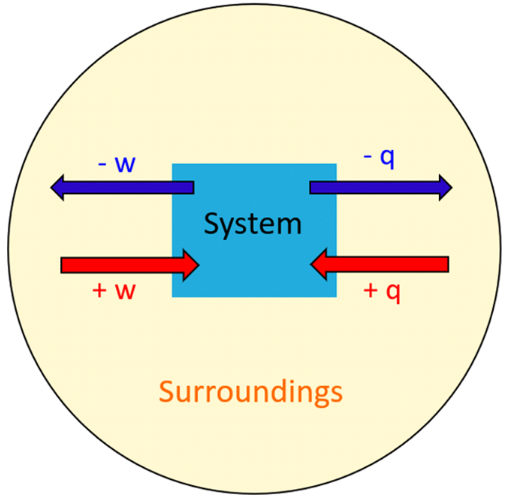
- If the reaction is exothermic (\( \mathrm{\Delta H < 0} \)), energy flows from the system to the surroundings.
- If the reaction is endothermic (\( \mathrm{\Delta H > 0} \)), energy flows from the surroundings into the system.
Key Relationships
- Exothermic Reaction: Reactants → Products + Energy
- Endothermic Reaction: Reactants + Energy → Products
- Heat flow continues until the system and surroundings reach the same temperature (thermal equilibrium).
Important Points
- Energy transfer during reaction affects the temperature of the surroundings.
- The sign of \( \mathrm{q} \) indicates the direction of energy flow:
- \( \mathrm{q > 0} \) → heat absorbed (endothermic)
- \( \mathrm{q < 0} \) → heat released (exothermic)
- At equilibrium: no further net energy exchange occurs.
Example :
When 50.0 mL of 1.0 M HCl reacts with 50.0 mL of 1.0 M NaOH in a calorimeter, the temperature rises from 25.0 °C to 31.5 °C. Determine the sign of \( \mathrm{\Delta H} \) and explain the energy transfer.
▶️ Answer / Explanation
Step 1: The temperature of the surroundings (solution) increases → heat is released by the reaction.
Step 2: Thus, the reaction is exothermic.
Step 3: Energy flow: \( \mathrm{q_{system} < 0} \) (system loses energy), \( \mathrm{q_{surroundings} > 0} \) (surroundings gain energy).
Final Answer: \( \mathrm{\Delta H < 0} \); the neutralization releases energy to the surroundings as heat.
Example :
When ammonium nitrate (\( \mathrm{NH_4NO_3} \)) dissolves in water, the solution becomes cold. Explain the direction of energy flow and the sign of \( \mathrm{\Delta H} \).
▶️ Answer / Explanation
Step 1: The solution cools → heat energy is absorbed from the surroundings by the dissolving salt.
Step 2: This makes the process endothermic.
Step 3: Energy flow: \( \mathrm{q_{system} > 0} \) (system gains energy), \( \mathrm{q_{surroundings} < 0} \) (surroundings lose energy).
Final Answer: \( \mathrm{\Delta H > 0} \); the reaction absorbs heat from the surroundings, lowering the temperature of the solution.
Chemical Potential Energy, Bond Changes, and Temperature Effects
The chemical potential energy of a substance is stored in the arrangement of its atoms and the strength of the bonds between them. During a chemical reaction, bonds in the reactants are broken and new bonds in the products are formed. The difference in these energies causes a change in the system’s total energy and affects the temperature of the surroundings.
Concept Explanation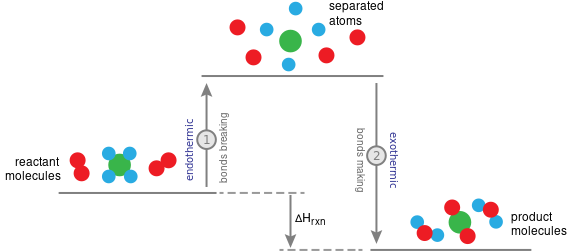
- Bonds Broken: Energy is absorbed (endothermic step).
- Bonds Formed: Energy is released (exothermic step).
- The overall enthalpy change of a reaction depends on the balance between these two energy quantities.
\( \mathrm{\Delta H = \sum E_{bonds\ broken} – \sum E_{bonds\ formed}} \)
If more energy is released forming new bonds than is absorbed breaking old ones → reaction is exothermic.
If more energy is absorbed breaking bonds than is released forming new ones → reaction is endothermic.
Key Relationships
![]()
- \( \mathrm{\Delta H < 0} \) → Exothermic → Energy released → Surroundings warm up.
- \( \mathrm{\Delta H > 0} \) → Endothermic → Energy absorbed → Surroundings cool down.
- Temperature changes in the surroundings are the observable result of energy transfer caused by these bond energy differences.
Important Points
- Bond-breaking requires energy (input), while bond-forming releases energy (output).
- The net change determines whether the overall process is endothermic or exothermic.
- The observed temperature change reflects the change in average kinetic energy of the particles in the surroundings.
Example :
Explain why the reaction between hydrogen gas and oxygen gas to form liquid water is exothermic. Identify which bonds are broken and formed, and describe the resulting energy change.
Given Reaction:
\( \mathrm{2H_2(g) + O_2(g) \rightarrow 2H_2O(l)} \)
▶️ Answer / Explanation
Step 1: Bonds broken: 2 H–H and 1 O=O → energy is absorbed to break these bonds.
Step 2: Bonds formed: 4 O–H → energy is released when new bonds form in water.
Step 3: The total energy released forming O–H bonds is greater than the energy absorbed breaking H–H and O=O bonds.
Step 4: Therefore, \( \mathrm{\Delta H < 0} \); the reaction is exothermic and releases energy as heat.
Final Answer: The formation of water releases energy to the surroundings, which increases their temperature due to higher kinetic energy of particles.
Example
The thermal decomposition of calcium carbonate occurs when it is heated strongly. Explain why this reaction is endothermic and describe the energy changes involved in bond breaking and formation.
Given Reaction:
\( \mathrm{CaCO_3(s) \rightarrow CaO(s) + CO_2(g)} \)
▶️ Answer / Explanation
Step 1: The strong ionic and covalent bonds in \( \mathrm{CaCO_3} \) must be broken → energy input is required.
Step 2: The energy absorbed to break these bonds is greater than the energy released when new bonds form in \( \mathrm{CaO} \) and \( \mathrm{CO_2} \).
Step 3: Because more energy is absorbed than released, the overall enthalpy change is positive.
Step 4: \( \mathrm{\Delta H > 0} \); the reaction is endothermic, and heat must be supplied continuously for it to proceed.
Final Answer: The decomposition of \( \mathrm{CaCO_3} \) absorbs heat energy — increasing the chemical potential energy of the products and lowering the temperature of the surroundings.
