AP Chemistry 6.7 Bond Enthalpies (Energies) Study Notes - New Syllabus Effective fall 2024
AP Chemistry 6.7 Bond Enthalpies (Energies) Study Notes.- New syllabus
AP Chemistry 6.7 Bond Enthalpies (Energies) Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Calculate the enthalpy change of a reaction based on the average bond energies of bonds broken and formed in the reaction.
Key Concepts:
- Bond Breaking, Bond Formation, and Potential Energy Changes
- Estimating Enthalpy Changes Using Average Bond Energies
Bond Breaking, Bond Formation, and Potential Energy Changes
During a chemical reaction, chemical bonds are broken and/or formed, and these events cause changes in the potential energy of the system.
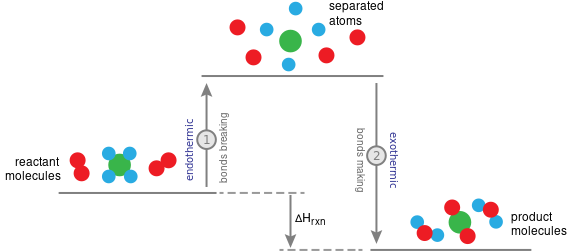
- Breaking bonds requires an input of energy (endothermic step) → increases potential energy.
- Forming bonds releases energy (exothermic step) → decreases potential energy.
- The balance between these two determines the overall energy change of the reaction.
\( \mathrm{\Delta H = \sum E_{bonds\ broken} – \sum E_{bonds\ formed}} \)
Interpretation:
- If more energy is released forming bonds than absorbed breaking bonds → exothermic reaction (\( \mathrm{\Delta H < 0} \)).
- If more energy is absorbed breaking bonds than released forming bonds → endothermic reaction (\( \mathrm{\Delta H > 0} \)).
Example:
In the reaction \( \mathrm{2H_2(g) + O_2(g) \rightarrow 2H_2O(l)} \), bonds in hydrogen and oxygen molecules are broken and new bonds form in water. Explain how these bond changes affect the potential energy of the system and determine whether the process is exothermic or endothermic.
▶️ Answer / Explanation
Step 1: Bonds broken → 2 H–H and 1 O=O → energy is absorbed.
Step 2: Bonds formed → 4 O–H → energy is released.
Step 3: Energy released forming O–H bonds is greater than energy absorbed breaking H–H and O=O bonds.
Step 4: The system’s potential energy decreases as energy is transferred to the surroundings.
Step 5: Therefore, \( \mathrm{\Delta H < 0} \); the reaction is exothermic.
Final Answer: The combustion of hydrogen decreases the system’s potential energy and releases energy to the surroundings as heat.
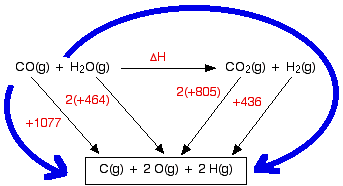
Estimating Enthalpy Changes Using Average Bond Energies
The average bond energy represents the energy required to break one mole of a specific type of bond in the gas phase. By summing all bond energies for bonds broken and formed, we can estimate the enthalpy change (\( \mathrm{\Delta H_{rxn}} \)) of a chemical reaction.
- The total energy required to break bonds in reactants can be estimated by adding their average bond energies.

- The total energy released when new bonds form in products can also be estimated.
- The difference between these two values gives the approximate enthalpy change of the reaction.
Formula:
\( \mathrm{\Delta H_{rxn} = \sum E_{bonds\ broken} – \sum E_{bonds\ formed}} \)
- \( \mathrm{E_{bonds\ broken}} \): total energy absorbed to break reactant bonds
- \( \mathrm{E_{bonds\ formed}} \): total energy released when product bonds form
Interpretation:
- If \( \mathrm{\sum E_{bonds\ formed} > \sum E_{bonds\ broken}} \) → \( \mathrm{\Delta H < 0} \) → Exothermic
- If \( \mathrm{\sum E_{bonds\ broken} > \sum E_{bonds\ formed}} \) → \( \mathrm{\Delta H > 0} \) → Endothermic
Key Idea: By comparing the total energy absorbed (bonds broken) and released (bonds formed), we can estimate whether a reaction is exothermic or endothermic. The difference in these energy changes represents the overall enthalpy change (\( \mathrm{\Delta H_{rxn}} \)).
Example:
Given average bond energies:
\( \mathrm{E(H–H) = 436\ kJ/mol,\ E(Cl–Cl) = 243\ kJ/mol,\ E(H–Cl) = 431\ kJ/mol.} \)
Estimate the enthalpy change for the reaction:
\( \mathrm{H_2(g) + Cl_2(g) \rightarrow 2HCl(g)} \)
▶️ Answer / Explanation
Step 1: Bonds broken (reactants):
- 1 × H–H = 436 kJ/mol
- 1 × Cl–Cl = 243 kJ/mol
Total energy absorbed = \( \mathrm{436 + 243 = 679\ kJ/mol} \)
Step 2: Bonds formed (products):
- 2 × H–Cl = \( \mathrm{2(431) = 862\ kJ/mol} \)
Total energy released = \( \mathrm{862\ kJ/mol} \)
Step 3: Calculate the overall enthalpy change:
\( \mathrm{\Delta H_{rxn} = 679 – 862 = -183\ kJ/mol} \)
Step 4: Interpretation:
- Energy released > energy absorbed → \( \mathrm{\Delta H < 0} \)
- The reaction is exothermic.
Final Answer: \( \mathrm{\Delta H_{rxn} = -183\ kJ/mol} \); the formation of H–Cl bonds releases more energy than is absorbed breaking H–H and Cl–Cl bonds.