AP Chemistry 6.9 Hess’s Law Study Notes - New Syllabus Effective fall 2024
AP Chemistry 6.9 Hess’s Law Study Notes- New syllabus
AP Chemistry 6.9 Hess’s Law Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship between the enthalpy of a chemical or physical process and the sum of the enthalpies of the individual steps.
Key Concepts:
- Representing Chemical or Physical Processes as a Sequence of Steps
- Conservation of Energy and the Basis of Hess’s Law
- Principles of Hess’s Law
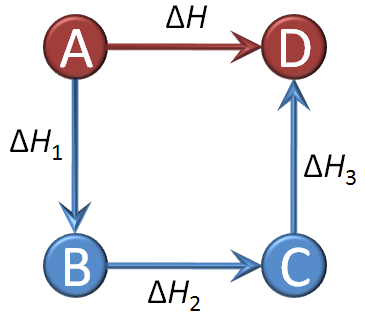
Representing Chemical or Physical Processes as a Sequence of Steps
Many chemical and physical processes can be represented as a series of smaller, individual steps. Each step has its own enthalpy change (\( \mathrm{\Delta H} \)), and the overall enthalpy change of the process is the sum of the enthalpy changes for all steps.
- Breaking a process into steps helps visualize energy flow and identify intermediate stages.
- Each step corresponds to a specific chemical or physical event (e.g., bond breaking, phase change, ionization, etc.).
- The total energy change of the entire process depends only on the initial and final states — not on the pathway (this concept forms the basis of Hess’s Law).
Key Relationship
\( \mathrm{\Delta H_{total} = \Delta H_1 + \Delta H_2 + \Delta H_3 + \cdots} \)
- The overall enthalpy change is the sum of all individual step enthalpy changes.
- Each step represents a specific physical or chemical transformation with a measurable \( \mathrm{\Delta H} \).
- This additive property of enthalpy allows indirect calculation of energy changes for reactions that are difficult to measure directly.
Illustrative Concept
For example, a phase change such as ice melting and then evaporating can be represented as two separate enthalpy steps:
\( \mathrm{H_2O(s) \xrightarrow{\Delta H_1} H_2O(l) \xrightarrow{\Delta H_2} H_2O(g)} \)
- \( \mathrm{\Delta H_1} \): enthalpy of fusion (melting)
- \( \mathrm{\Delta H_2} \): enthalpy of vaporization (evaporation)
- Total \( \mathrm{\Delta H_{total} = \Delta H_1 + \Delta H_2} \)
Concept Summary
- Each step of a process contributes a specific energy change (\( \mathrm{\Delta H_i} \)).
- The total enthalpy change equals the algebraic sum of all step changes.
- Used widely in thermochemistry and to construct enthalpy (energy) diagrams and apply Hess’s Law.
Example :
Represent the process of converting 1 mol of ice at 0 °C to steam at 100 °C as a sequence of steps, and write the overall enthalpy relationship.
▶️ Answer / Explanation
Step 1: Ice melts to liquid water at 0 °C.
\( \mathrm{H_2O(s) \rightarrow H_2O(l)} \quad \Delta H_1 = \Delta H_{fus} \)
Step 2: Liquid water warms from 0 °C to 100 °C.
\( \mathrm{H_2O(l) \rightarrow H_2O(l)_{100°C}} \quad \Delta H_2 = mC_p\Delta T \)
Step 3: Liquid water vaporizes at 100 °C.
\( \mathrm{H_2O(l) \rightarrow H_2O(g)} \quad \Delta H_3 = \Delta H_{vap} \)
Overall Process:
\( \mathrm{H_2O(s) \rightarrow H_2O(g)} \)
Total Enthalpy Change:
\( \mathrm{\Delta H_{total} = \Delta H_{fus} + mC_p\Delta T + \Delta H_{vap}} \)
Interpretation: Each stage contributes a measurable energy change. The sum of all these step enthalpies gives the total energy required to convert solid ice into gaseous water (steam).
Example :
Represent the formation of \( \mathrm{CO_2(g)} \) from graphite and oxygen as a sequence of steps and determine the overall enthalpy relationship.
▶️ Answer / Explanation
Step 1: Convert graphite to carbon monoxide.
\( \mathrm{C_{(graphite)} + \dfrac{1}{2}O_2(g) \rightarrow CO(g)} \quad \Delta H_1 = -110.5\ kJ/mol \)
Step 2: Oxidize carbon monoxide to carbon dioxide.
\( \mathrm{CO(g) + \dfrac{1}{2}O_2(g) \rightarrow CO_2(g)} \quad \Delta H_2 = -283.0\ kJ/mol \)
Overall Reaction:
\( \mathrm{C_{(graphite)} + O_2(g) \rightarrow CO_2(g)} \)
Total Enthalpy Change:
\( \mathrm{\Delta H_{total} = \Delta H_1 + \Delta H_2 = (-110.5) + (-283.0) = -393.5\ kJ/mol} \)
Final Answer: \( \mathrm{\Delta H_{total} = -393.5\ kJ/mol} \); representing the direct formation of \( \mathrm{CO_2(g)} \) as the sum of two exothermic steps.
Conservation of Energy and the Basis of Hess’s Law
According to the First Law of Thermodynamics, energy can neither be created nor destroyed — it can only be transformed or transferred. In chemical processes, this means that the total energy of a system and its surroundings remains constant. When a process occurs in multiple steps, each step involves its own thermal energy change (\( \mathrm{q} \)) or enthalpy change (\( \mathrm{\Delta H} \)). Because energy is conserved, the net energy change for the overall process equals the sum of the energy changes for the individual steps.
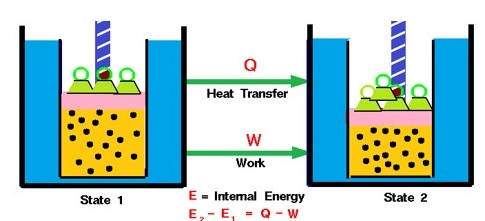
- Each step may absorb or release thermal energy.
- Energy changes in one step can be canceled or combined with others.
- At constant pressure, these thermal energy transfers correspond to changes in enthalpy.
Key Relationship (Hess’s Law Basis)
\( \mathrm{\Delta H_{overall} = \Delta H_1 + \Delta H_2 + \Delta H_3 + \cdots} \)
- This relationship expresses the additivity of enthalpy changes.

- Since enthalpy is a state function, it depends only on initial and final states — not on the reaction pathway.
- Thus, the enthalpy change for a complex reaction can be found by summing the enthalpy changes of multiple simpler reactions.
Summary:
- Total Energy Conservation: Energy released in one step can be used in another — total remains constant.
- Thermal Energy Transfer: Each reaction step transfers heat to or from surroundings.
- Overall Enthalpy Change: Equals the sum of individual step enthalpies at constant pressure.
Example:
The overall reaction for forming carbon dioxide from graphite can occur through two separate steps:
Step 1: \( \mathrm{C_{(graphite)} + \dfrac{1}{2}O_2(g) \rightarrow CO(g)} \) \( \mathrm{\Delta H_1 = -110.5\ kJ/mol} \)
Step 2: \( \mathrm{CO(g) + \dfrac{1}{2}O_2(g) \rightarrow CO_2(g)} \) \( \mathrm{\Delta H_2 = -283.0\ kJ/mol} \)
Overall Reaction: \( \mathrm{C_{(graphite)} + O_2(g) \rightarrow CO_2(g)} \)
▶️ Answer / Explanation
Step 1: Apply Hess’s Law — add the two enthalpy changes:
\( \mathrm{\Delta H_{overall} = \Delta H_1 + \Delta H_2} \)
Step 2: Substitute the values:
\( \mathrm{\Delta H_{overall} = (-110.5) + (-283.0) = -393.5\ kJ/mol} \)
Step 3: Interpretation:
- Total enthalpy change equals the sum of step enthalpies.
- Negative value → exothermic process (heat released).
Final Answer: \( \mathrm{\Delta H_{overall} = -393.5\ kJ/mol} \); energy conservation ensures that the total heat released in the two-step pathway equals the heat released in the single-step direct reaction.
Principles of Hess’s Law
Hess’s Law states that the overall enthalpy change for a reaction is the same, regardless of the number of steps or the path taken, because enthalpy is a state function. This means the total change in enthalpy (\( \mathrm{\Delta H} \)) depends only on the initial and final states, not on the route between them.
- If a chemical equation can be expressed as the sum of two or more other equations, then the total enthalpy change is the sum of the enthalpy changes for each individual reaction.
- This allows us to determine enthalpy changes indirectly by combining known thermochemical equations.
Mathematical Relationship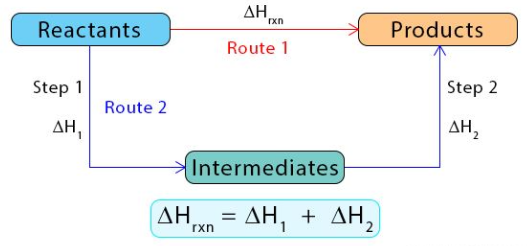
\( \mathrm{\Delta H_{overall} = \sum \Delta H_{steps}} \)
To correctly apply Hess’s Law, it’s important to follow these three essential rules:
Principles of Hess’s Law
Reversing a Reaction:
When a chemical equation is reversed, the sign of its enthalpy change is also reversed.
\( \mathrm{A \rightarrow B,\ \Delta H = +x\ kJ} \Rightarrow \mathrm{B \rightarrow A,\ \Delta H = -x\ kJ} \)
Multiplying a Reaction by a Factor:
When the entire reaction is multiplied by a constant \( \mathrm{c} \), the enthalpy change is also multiplied by that same factor.
\( \mathrm{c(A \rightarrow B)} \Rightarrow \mathrm{\Delta H_{new} = c \times \Delta H_{original}} \)
Adding Reactions:
When two or more reactions are added to produce an overall reaction, their enthalpy changes are also added.
\( \mathrm{(A \rightarrow B, \ \Delta H_1) + (B \rightarrow C, \ \Delta H_2)} \Rightarrow \mathrm{A \rightarrow C, \ \Delta H_{total} = \Delta H_1 + \Delta H_2} \)
Key Note:
- Hess’s Law follows directly from the law of conservation of energy.
- It allows calculation of enthalpy changes that are experimentally difficult to measure directly.
- Each manipulation of an equation (reversal, scaling, addition) affects \( \mathrm{\Delta H} \) accordingly.
Example :
If the reaction \( \mathrm{H_2(g) + Cl_2(g) \rightarrow 2HCl(g)} \) has \( \mathrm{\Delta H = -184.6\ kJ/mol} \), what is the enthalpy change for the reverse reaction?
▶️ Answer / Explanation
Step 1: Reverse the reaction: \( \mathrm{2HCl(g) \rightarrow H_2(g) + Cl_2(g)} \).
Step 2: Reverse the sign of \( \mathrm{\Delta H} \).
\( \mathrm{\Delta H = +184.6\ kJ/mol} \)
Final Answer: When the direction of a reaction is reversed, the magnitude of \( \mathrm{\Delta H} \) stays the same but its sign changes.
Example :
If \( \mathrm{N_2(g) + 3H_2(g) \rightarrow 2NH_3(g)} \) has \( \mathrm{\Delta H = -92.2\ kJ} \), what is \( \mathrm{\Delta H} \) for producing 4 mol of \( \mathrm{NH_3(g)} \)?
▶️ Answer / Explanation
Step 1: Multiply the entire reaction by 2 to produce 4 mol of ammonia.
\( \mathrm{2N_2(g) + 6H_2(g) \rightarrow 4NH_3(g)} \)
Step 2: Multiply \( \mathrm{\Delta H} \) by the same factor.
\( \mathrm{\Delta H = 2(-92.2) = -184.4\ kJ} \)
Final Answer: The enthalpy change doubles when the reaction is doubled; \( \mathrm{\Delta H = -184.4\ kJ} \).
Example :
Given the following reactions, determine \( \mathrm{\Delta H_{overall}} \) for \( \mathrm{C_{(graphite)} + O_2(g) \rightarrow CO_2(g)} \):
Step 1: \( \mathrm{C_{(graphite)} + \dfrac{1}{2}O_2(g) \rightarrow CO(g)} \) \( \mathrm{\Delta H_1 = -110.5\ kJ/mol} \)
Step 2: \( \mathrm{CO(g) + \dfrac{1}{2}O_2(g) \rightarrow CO_2(g)} \) \( \mathrm{\Delta H_2 = -283.0\ kJ/mol} \)
▶️ Answer / Explanation
Step 1: Add the two equations to cancel the intermediate \( \mathrm{CO(g)} \).
\( \mathrm{C_{(graphite)} + O_2(g) \rightarrow CO_2(g)} \)
Step 2: Add the enthalpy changes:
\( \mathrm{\Delta H_{overall} = \Delta H_1 + \Delta H_2 = (-110.5) + (-283.0) = -393.5\ kJ/mol} \)
Final Answer: \( \mathrm{\Delta H_{overall} = -393.5\ kJ/mol} \); total enthalpy change equals the sum of enthalpies of all individual steps.