AP Chemistry 7.10 Reaction Quotient and Le Chateilier’s Principle Study Notes - New Syllabus Effective fall 2024
AP Chemistry 7.10 Reaction Quotient and Le Chateilier’s Principle Study Notes- New syllabus
AP Chemistry 7.10 Reaction Quotient and Le Chateilier’s Principle Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationships between Q, K, and the direction in which a reversible reaction will proceed to reach equilibrium.
Key Concepts:
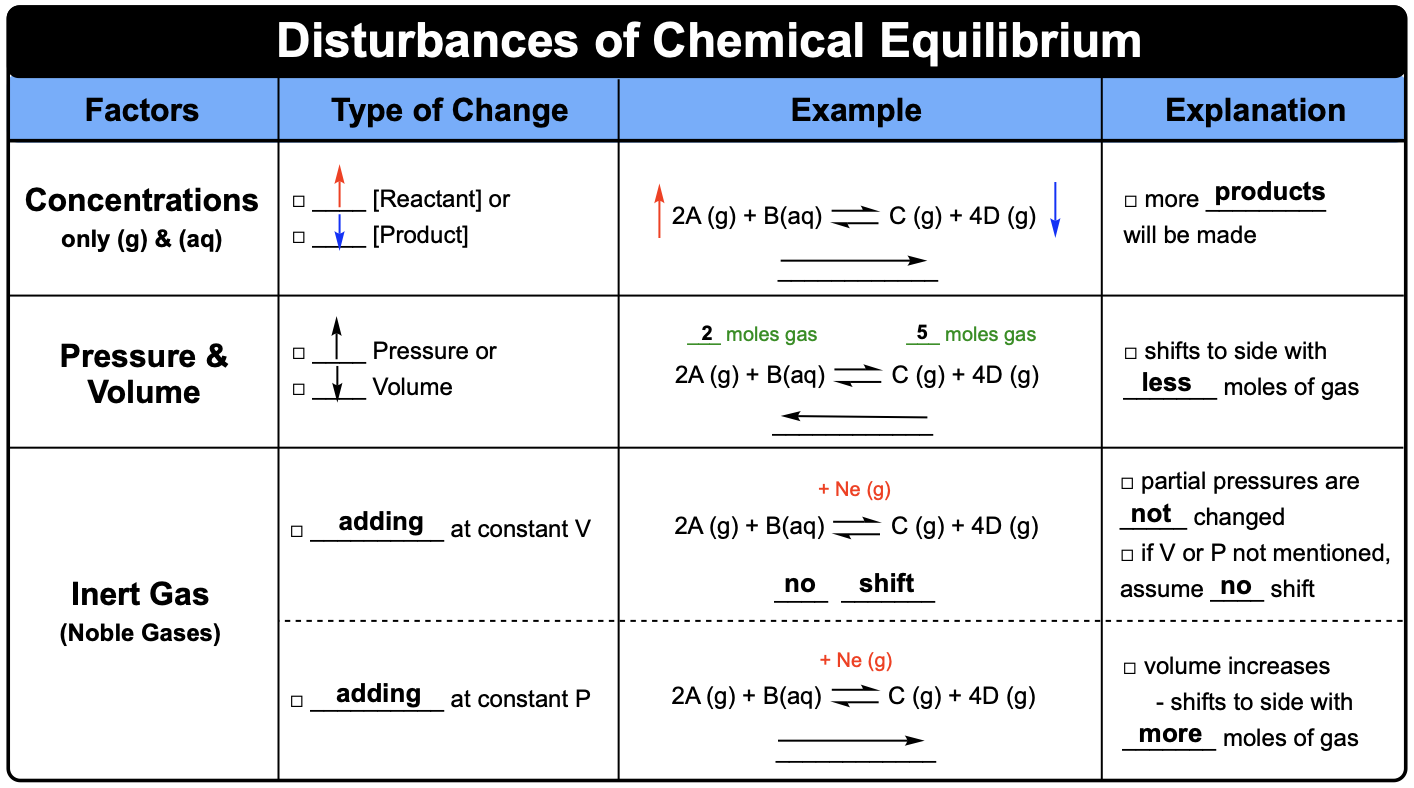
- How a Disturbance Causes Q ≠ K
- Disturbances Affecting Q vs. K
How a Disturbance Causes Q ≠ K
At equilibrium, a system satisfies \( \mathrm{Q = K} \). When a disturbance (such as changing concentration, pressure, or temperature) is applied, the system temporarily moves out of equilibrium so that \( \mathrm{Q ≠ K} \). The system then shifts in the direction that restores equality between \( \mathrm{Q} \) and \( \mathrm{K} \), establishing a new equilibrium state.
Key Idea:
- Equilibrium position shifts because a disturbance changes relative product/reactant ratios.
- The system always acts to re-establish Q = K.
- Direction of shift depends on whether \( \mathrm{Q} \) becomes smaller or larger than \( \mathrm{K} \).
Mathematical Relationship:
For a general reaction \( \mathrm{aA + bB ⇄ cC + dD} \):
\( \mathrm{Q_c = \dfrac{[C]^c [D]^d}{[A]^a [B]^b}} \)
- If \( \mathrm{Q < K} \): forward reaction proceeds (→ products).
- If \( \mathrm{Q > K} \): reverse reaction proceeds (→ reactants).
Example :
Reaction: \( \mathrm{N_2(g) + 3H_2(g) ⇄ 2NH_3(g)} \)
At equilibrium: \( \mathrm{K_c = 6.0 \times 10^5} \)
Disturbance: Additional \( \mathrm{H_2} \) gas is added.
How does the system respond?
▶️ Answer / Explanation
Step 1: Adding \( \mathrm{H_2} \) increases the denominator in \( \mathrm{Q_c = \dfrac{[NH_3]^2}{[N_2][H_2]^3}} \).
Step 2: Thus, \( \mathrm{Q_c < K_c} \).
Step 3: The system shifts right (→) to produce more \( \mathrm{NH_3} \) until Q increases back to equal K.
Final Answer: Reaction shifts toward products to restore equilibrium (\( \mathrm{Q = K} \)).
Disturbances Affecting Q vs. K
Some disturbances (like changing concentration or pressure) alter the value of \( \mathrm{Q} \) but not \( \mathrm{K} \). A change in temperature, however, alters the value of \( \mathrm{K} \) itself, because temperature affects the reaction’s enthalpy and equilibrium constant.

Key Distinctions:
| Type of Disturbance | Changes Q? | Changes K? | System Response |
|---|---|---|---|
| Change in concentration | Yes | No | Shifts to restore Q = K |
| Change in pressure (gas) | Yes | No | Shifts toward fewer or more gas moles |
| Change in temperature | Yes (temporarily) | Yes | New K value established for new T |
Mathematical Note:
- When temperature changes, the value of K changes according to the reaction’s enthalpy:
Exothermic: \( \mathrm{\text{T↑} \Rightarrow K↓} \) Endothermic: \( \mathrm{\text{T↑} \Rightarrow K↑} \)
Example :
Reaction: \( \mathrm{2NO_2(g) ⇄ N_2O_4(g)} \), ΔH = −58 kJ (exothermic)
Predict how \( \mathrm{K_c} \) and equilibrium composition change with temperature increase.
▶️ Answer / Explanation
Step 1: Exothermic → heat acts as a product: \( \mathrm{2NO_2 ⇄ N_2O_4 + heat} \).
Step 2: Increasing temperature adds heat → shifts left (endothermic direction).
Step 3: New equilibrium forms with more \( \mathrm{NO_2} \) and less \( \mathrm{N_2O_4} \); \( \mathrm{K_c} \) decreases.
Final Answer: \( \mathrm{K_c↓} \), equilibrium shifts left, brown color intensifies.
Example :
Reaction: \( \mathrm{H_2(g) + I_2(g) ⇄ 2HI(g)} \)
Disturbance: Adding \( \mathrm{HI} \) gas.
Which direction will the reaction shift to re-establish equilibrium?
▶️ Answer / Explanation
Step 1: Adding \( \mathrm{HI} \) increases the numerator of \( \mathrm{Q_c = \dfrac{[HI]^2}{[H_2][I_2]}} \).
Step 2: Now \( \mathrm{Q_c > K_c} \).
Step 3: The system shifts left (←) to consume excess \( \mathrm{HI} \), forming more \( \mathrm{H_2} \) and \( \mathrm{I_2} \).
Final Answer: \( \mathrm{Q} \) returns to equal \( \mathrm{K} \); new equilibrium established with lower \( \mathrm{HI} \).
