AP Chemistry 7.12 Common-Ion Effect Study Notes - New Syllabus Effective fall 2024
AP Chemistry 7.12 Common-Ion Effect Study Notes- New syllabus
AP Chemistry 7.12 Common-Ion Effect Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Identify the solubility of a salt, and/or the value of Ksp for the salt, based on the concentration of a common ion already present in solution.
Key Concepts:
- Common-Ion Effect
The Effect of a Common Ion on Solubility
The common-ion effect occurs when a salt is dissolved in a solution that already contains one of the ions that the salt produces upon dissolution. Because the ion is already present, the equilibrium shifts to favor the undissolved solid form of the salt, thereby reducing its solubility.
This effect can be explained both qualitatively using Le Châtelier’s Principle and quantitatively using the solubility product constant \( \mathrm{K_{sp}} \).
General Dissolution Reaction: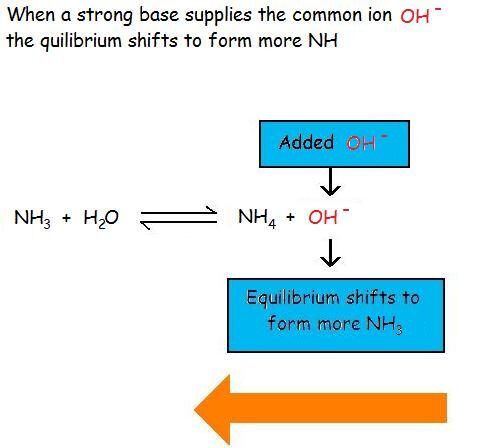
\( \mathrm{A_{m}B_{n(s)} ⇄ mA^{n+}(aq) + nB^{m-}(aq)} \)
Solubility Product:
\( \mathrm{K_{sp} = [A^{n+}]^m [B^{m-}]^n} \)
When a Common Ion Is Present:
- Suppose the solution already contains \( \mathrm{A^{n+}} \) from another source.
- The initial concentration of \( \mathrm{A^{n+}} \) increases → equilibrium shifts left.
- Result: Less \( \mathrm{A_{m}B_{n}} \) dissolves, and solubility (s) decreases.
Explanation Using Le Châtelier’s Principle:
Adding a common ion increases the concentration of a product species in the dissolution equilibrium. To counteract this disturbance, the system shifts toward the reactant side (solid salt), resulting in precipitation or reduced solubility.
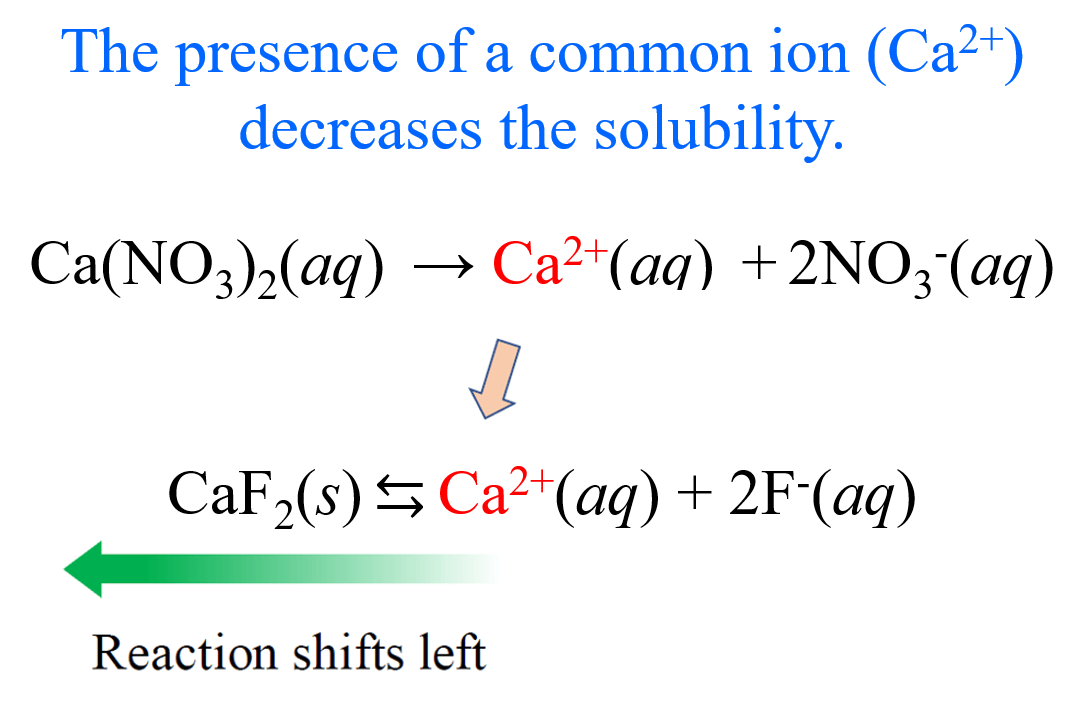
Increased [common ion] → shift left → decreased solubility.
Mathematical Expression (Example for 1:1 Salt):
For \( \mathrm{MX(s) ⇄ M^+(aq) + X^-(aq)} \),
\( \mathrm{K_{sp} = [M^+][X^-]} \)
If a common ion \( \mathrm{X^-} \) is present at concentration \( \mathrm{c} \), and additional \( \mathrm{s} \) moles of salt dissolve, then:
\( \mathrm{K_{sp} = (s)(c + s) ≈ s(c)} \) (since \( \mathrm{s \ll c} \))
Thus, \( \mathrm{s = \dfrac{K_{sp}}{c}} \), showing that solubility decreases as \( \mathrm{[common\ ion]} \) increases.
Key Notes:
- The presence of a common ion reduces the solubility of a slightly soluble salt.
- The equilibrium shift (left) can be explained by Le Châtelier’s Principle.
- Mathematically, solubility \( \mathrm{s} \) decreases in inverse proportion to the concentration of the common ion.
- \( \mathrm{K_{sp}} \) remains constant for a given temperature — only solubility changes.
Important Relationship:
\( \mathrm{s_{new} = \dfrac{K_{sp}}{[common\ ion]}} \) (for simple 1:1 salts)
Thus, increasing the common-ion concentration decreases the solubility proportionally.
Example:
Reaction: \( \mathrm{PbCl_2(s) ⇄ Pb^{2+}(aq) + 2Cl^-(aq)} \)
Given: \( \mathrm{K_{sp} = 1.6 \times 10^{-5}} \); the solution already contains \( \mathrm{[Cl^-] = 0.10\ M} \).
What is the solubility (\( \mathrm{s} \)) of \( \mathrm{PbCl_2} \) in this solution?
▶️ Answer / Explanation
Step 1: Write the equilibrium concentrations:
- \( \mathrm{[Pb^{2+}] = s} \)
- \( \mathrm{[Cl^-] = 0.10 + 2s ≈ 0.10} \) (since \( \mathrm{s \ll 0.10} \))
Step 2: Write the \( \mathrm{K_{sp}} \) expression:
\( \mathrm{K_{sp} = [Pb^{2+}][Cl^-]^2 = (s)(0.10)^2} \)
Step 3: Solve for \( \mathrm{s} \):
\( \mathrm{s = \dfrac{1.6 \times 10^{-5}}{(0.10)^2} = 1.6 \times 10^{-3}\ M} \)
Step 4: Compare with solubility in pure water:
- In pure water: \( \mathrm{s = \sqrt[3]{\dfrac{K_{sp}}{4}} = 1.6 \times 10^{-2}\ M} \)
- With common ion: \( \mathrm{s = 1.6 \times 10^{-3}\ M} \)
Final Answer: Solubility decreases tenfold due to the presence of \( \mathrm{Cl^-} \) ions.
Example :
Which salt will be less soluble in 0.10 M NaF solution — \( \mathrm{CaF_2} \) or \( \mathrm{AgF} \)?
▶️ Answer / Explanation
Step 1: The solution already contains \( \mathrm{F^-} \) from NaF.
Step 2: Both salts produce \( \mathrm{F^-} \) upon dissolving, but \( \mathrm{CaF_2} \) produces two per formula unit → affected more strongly.
Step 3: The common-ion effect suppresses \( \mathrm{CaF_2} \) solubility more.
Final Answer: \( \mathrm{CaF_2} \) is much less soluble than \( \mathrm{AgF} \) in 0.10 M NaF solution.
