AP Chemistry 7.2 Direction of Reversible Reactions Study Notes - New Syllabus Effective fall 2024
AP Chemistry 7.2 Direction of Reversible Reactions Study Notes- New syllabus
AP Chemistry 7.2 Direction of Reversible Reactions Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship between the direction in which a reversible reaction proceeds and the relative rates of the forward and reverse reactions.
Key Concepts:
- Reversible Reactions
Relationship Between Reaction Direction and Relative Rates
A reversible reaction can proceed in both directions — forward (reactants forming products) and reverse (products reforming reactants). The net direction in which the reaction proceeds depends on which rate — forward or reverse — is greater at a given moment.
Key Relationships:
- If \( \mathrm{Rate_{forward} > Rate_{reverse}} \), the system experiences a net conversion of reactants to products.
- If \( \mathrm{Rate_{reverse} > Rate_{forward}} \), the system experiences a net conversion of products to reactants.
- When \( \mathrm{Rate_{forward} = Rate_{reverse}} \), the system has reached equilibrium, and no net change in concentrations occurs.
Mathematical Representation:
\( \mathrm{aA + bB ⇄ cC + dD} \)
\( \mathrm{Rate_{forward} = k_f [A]^a [B]^b} \)
\( \mathrm{Rate_{reverse} = k_r [C]^c [D]^d} \)
At equilibrium: \( \mathrm{k_f [A]^a [B]^b = k_r [C]^c [D]^d} \)
Therefore, the equilibrium constant can be expressed as:
\( \mathrm{K = \dfrac{k_f}{k_r}} \)
Conceptual Understanding:
- At the start of a reaction, only reactants are present, so \( \mathrm{Rate_{forward}} \) is high and \( \mathrm{Rate_{reverse}} \) is near zero.
- As products accumulate, \( \mathrm{Rate_{reverse}} \) increases because more products are available to reform reactants.
- Eventually, both rates become equal — the system has reached dynamic equilibrium.
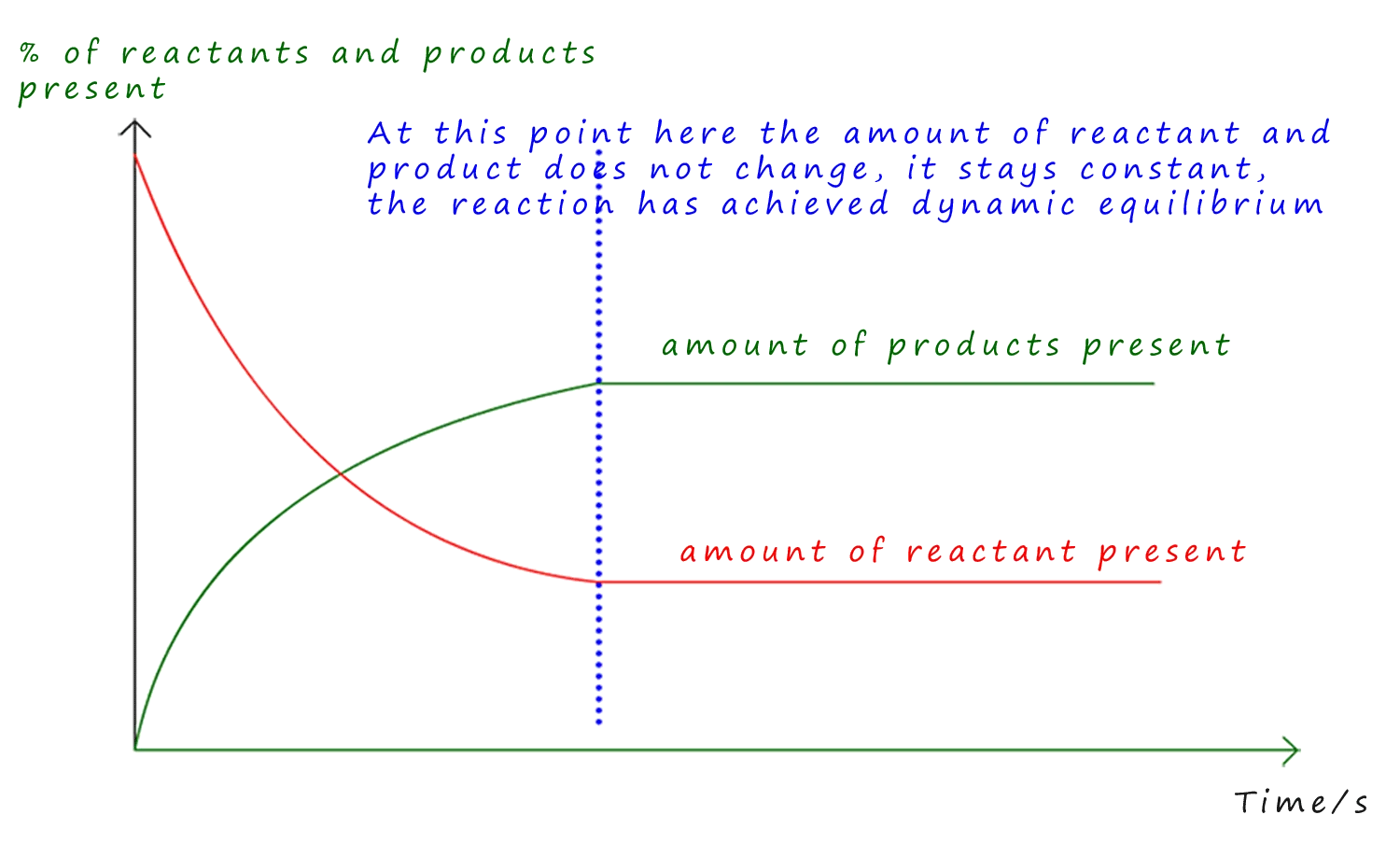
Graphical Interpretation:
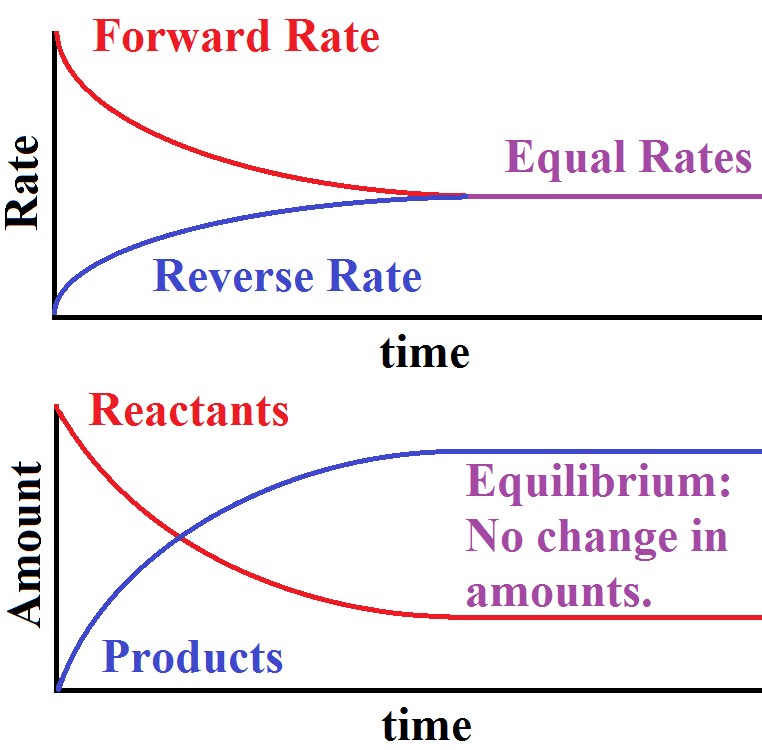
- Rate vs. Time: Forward rate starts high and decreases; reverse rate starts low and increases until both meet at equilibrium.
- Concentration vs. Time: Reactant concentration decreases while product concentration increases until both flatten at equilibrium.
The direction of a reversible reaction at any given moment depends on which rate is greater. Equilibrium is reached when the rates are equal the hallmark of a dynamic equilibrium.
Example:
\( \mathrm{2NO_2(g) ⇄ N_2O_4(g)} \)
Explain the relationship between the direction of the reaction and the relative rates of the forward and reverse processes as equilibrium is approached.
▶️ Answer / Explanation
Step 1: Initially, only \( \mathrm{NO_2} \) is present, so \( \mathrm{Rate_{forward}} \) is large and \( \mathrm{Rate_{reverse}} \) is nearly zero. The reaction proceeds toward forming \( \mathrm{N_2O_4} \).
Step 2: As \( \mathrm{N_2O_4} \) forms, its concentration increases, making \( \mathrm{Rate_{reverse}} \) increase.
Step 3: Over time, \( \mathrm{Rate_{forward}} \) decreases (less \( \mathrm{NO_2} \) available), while \( \mathrm{Rate_{reverse}} \) increases (more \( \mathrm{N_2O_4} \) present).
Step 4: When both rates become equal, \( \mathrm{Rate_{forward} = Rate_{reverse}} \), equilibrium is reached. No net change in color or concentration occurs, even though both reactions continue at equal rates.
Final Answer: Before equilibrium, the reaction direction is determined by the faster rate. At equilibrium, equal forward and reverse rates result in no net observable change.
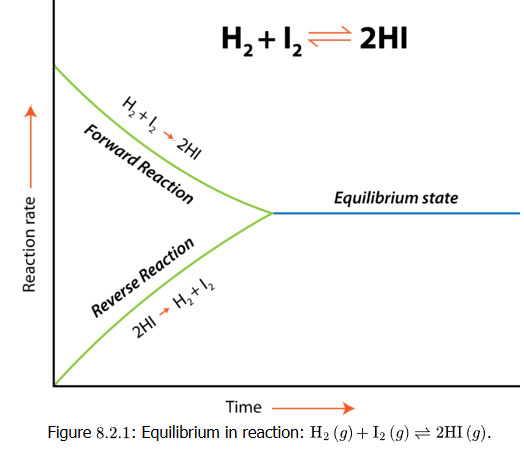
Example :
\( \mathrm{H_2(g) + I_2(g) ⇄ 2HI(g)} \)
Describe what happens to the rates of the forward and reverse reactions as the system moves toward equilibrium starting from only \( \mathrm{H_2} \) and \( \mathrm{I_2} \).
▶️ Answer / Explanation
Step 1: Initially, only \( \mathrm{H_2} \) and \( \mathrm{I_2} \) are present, so \( \mathrm{Rate_{forward}} \) is high and \( \mathrm{Rate_{reverse}} = 0 \).
Step 2: As \( \mathrm{HI} \) forms, the reverse reaction (\( \mathrm{2HI ⇄ H_2 + I_2} \)) begins, so \( \mathrm{Rate_{reverse}} \) starts to increase.
Step 3: Over time, \( \mathrm{Rate_{forward}} \) decreases (reactants are consumed) and \( \mathrm{Rate_{reverse}} \) increases (products accumulate).
Step 4: When \( \mathrm{Rate_{forward} = Rate_{reverse}} \), equilibrium is reached — the system shows no net change.
Final Answer: The reaction proceeds forward initially, then slows as the reverse rate increases. Equilibrium is achieved when both rates become equal.
Example :
\( \mathrm{AgCl(s) ⇄ Ag^+(aq) + Cl^-(aq)} \)
How do the relative rates of dissolution and precipitation determine whether more solid \( \mathrm{AgCl} \) dissolves or forms?
▶️ Answer / Explanation
Step 1: Initially, in pure water, there are no \( \mathrm{Ag^+} \) or \( \mathrm{Cl^-} \) ions, so only the forward reaction (dissolution) occurs rapidly.
Step 2: As ions build up, the reverse reaction (precipitation) rate increases because ions recombine to form \( \mathrm{AgCl(s)} \).
Step 3: Eventually, both rates become equal:
\( \mathrm{Rate_{dissolution} = Rate_{precipitation}} \)
At this point, the amount of solid \( \mathrm{AgCl} \) remains constant, though ions continue to exchange between solid and solution.
Final Answer: The net direction of change depends on the relative rates of dissolution and precipitation. Equilibrium is reached when both occur at equal rates — the hallmark of a dynamic equilibrium.
