AP Chemistry 7.3 Reaction Quotient and Equilibrium Constant Study Notes - New Syllabus Effective fall 2024
AP Chemistry 7.3 Reaction Quotient and Equilibrium Constant Study Notes- New syllabus
AP Chemistry 7.3 Reaction Quotient and Equilibrium Constant Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Represent the reaction quotient Qc or Qp, for a reversible reaction, and the corresponding equilibrium expressions Kc =Qc or Kp =Qp.
Key Concepts:
- The Reaction Quotient
- Calculating the Equilibrium Constant
- Magnitude of the Equilibrium Constant
- Manipulating the Equilibrium Constant
Representing the Reaction Quotient and Equilibrium Constant Expressions
The reaction quotient (\( \mathrm{Q} \)) is a mathematical expression that describes the relative amounts of reactants and products at a given moment — not necessarily at equilibrium. It helps determine whether a system is at equilibrium and in which direction it will shift to reach it.
Key Idea:
- At any instant during a reaction, the reaction quotient \( \mathrm{Q} \) can be calculated using the same form as the equilibrium constant \( \mathrm{K} \).
- At equilibrium, \( \mathrm{Q = K} \).
- If the system is not at equilibrium, comparing \( \mathrm{Q} \) and \( \mathrm{K} \) indicates the direction of shift:
- \( \mathrm{Q < K} \): Reaction shifts forward (toward products).
- \( \mathrm{Q > K} \): Reaction shifts backward (toward reactants).
- \( \mathrm{Q = K} \): System is at equilibrium.
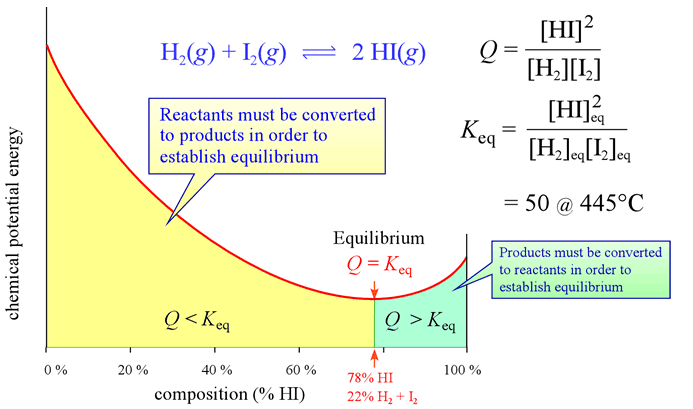
Equilibrium Law of Mass Action:
For a general reaction:
\( \mathrm{aA + bB ⇄ cC + dD} \)
The expressions for \( \mathrm{Q_c} \) and \( \mathrm{K_c} \) are given by:
\( \mathrm{Q_c = \dfrac{[C]^c [D]^d}{[A]^a [B]^b}} \)
\( \mathrm{K_c = \dfrac{[C]^c [D]^d}{[A]^a [B]^b}} \)
Similarly, for gas-phase reactions using partial pressures:
\( \mathrm{Q_p = \dfrac{(P_C)^c (P_D)^d}{(P_A)^a (P_B)^b}} \)
\( \mathrm{K_p = \dfrac{(P_C)^c (P_D)^d}{(P_A)^a (P_B)^b}} \)
Note: The only difference between \( \mathrm{Q} \) and \( \mathrm{K} \) is that \( \mathrm{Q} \) can be determined at any point in the reaction, while \( \mathrm{K} \) applies only when the system is at equilibrium.
Relationship Between \( \mathrm{K_p} \) and \( \mathrm{K_c} \):
\( \mathrm{K_p = K_c (RT)^{\Delta n}} \)
where \( \mathrm{\Delta n = (moles\ of\ gaseous\ products) – (moles\ of\ gaseous\ reactants)} \)
| Condition | Comparison | Reaction Shift |
|---|---|---|
| More reactants than equilibrium | \( \mathrm{Q < K} \) | Forward (→) |
| More products than equilibrium | \( \mathrm{Q > K} \) | Reverse (←) |
| System at equilibrium | \( \mathrm{Q = K} \) | No net shift |
Example
Reaction: \( \mathrm{N_2(g) + 3H_2(g) ⇄ 2NH_3(g)} \)
Write expressions for \( \mathrm{Q_c} \) and \( \mathrm{Q_p} \), and explain what happens if \( \mathrm{Q_p < K_p} \).
▶️ Answer / Explanation
Step 1: For concentration form:
\( \mathrm{Q_c = \dfrac{[NH_3]^2}{[N_2][H_2]^3}} \)
Step 2: For partial pressure form:
\( \mathrm{Q_p = \dfrac{(P_{NH_3})^2}{(P_{N_2})(P_{H_2})^3}} \)
Step 3: If \( \mathrm{Q_p < K_p} \), there are too few products and too many reactants. The reaction shifts forward to produce more \( \mathrm{NH_3} \) and reach equilibrium.
Final Answer: \( \mathrm{Q_p < K_p} \) → forward shift (products favored).
Example
Reaction: \( \mathrm{2SO_2(g) + O_2(g) ⇄ 2SO_3(g)} \)
\( \mathrm{K_c = 4.0 \times 10^{24}} \) at 700 K, and initial concentrations \( \mathrm{[SO_2] = [O_2] = [SO_3] = 0.10\ M} \).
▶️ Answer / Explanation
Step 1: Calculate \( \mathrm{Q_c} \):
\( \mathrm{Q_c = \dfrac{[SO_3]^2}{[SO_2]^2 [O_2]} = \dfrac{(0.10)^2}{(0.10)^2(0.10)} = 10} \)
Step 2: Compare \( \mathrm{Q_c} \) and \( \mathrm{K_c} \): \( \mathrm{Q_c = 10 \ll K_c = 4.0 \times 10^{24}} \).
Step 3: Since \( \mathrm{Q_c < K_c} \), the forward reaction is favored — the system shifts to produce more \( \mathrm{SO_3} \).
Final Answer: The reaction proceeds forward until equilibrium is reached.
Exclusion of Solids and Pure Liquids from Reaction Quotient Expressions
In equilibrium expressions, the reaction quotient (Q) and equilibrium constant (K) include only species whose concentrations or partial pressures change with the progress of the reaction. Pure solids and pure liquids are excluded because their densities — and therefore their molar concentrations — remain constant regardless of the amount present.
Key Reasoning:
- The concentration of a solid or liquid depends only on its density and molar mass, which are constants at a given temperature.
- Adding or removing a pure solid or pure liquid does not change its concentration and therefore does not affect equilibrium composition.
- Only gaseous and aqueous species (whose concentrations vary) are included in \( \mathrm{Q} \) or \( \mathrm{K} \).
Conceptual Rule:
| Phase of Substance | Included in Q or K? | Reason |
|---|---|---|
| Gas (g) | Yes | Variable partial pressure / concentration |
| Aqueous (aq) | Yes | Variable concentration in solution |
| Liquid (l) | No | Constant density → constant concentration |
| Solid (s) | No | Constant density → constant concentration |
Mathematical Representation:
For a general heterogeneous reaction:
\( \mathrm{aA_{(s)} + bB_{(g)} ⇄ cC_{(g)} + dD_{(l)}} \)
The equilibrium expression includes only the gas or aqueous species:
\( \mathrm{K = \dfrac{[C]^c}{[B]^b}} \)
The solid \( \mathrm{A_{(s)}} \) and liquid \( \mathrm{D_{(l)}} \) are omitted.
Example :
Reaction: \( \mathrm{CaCO_3(s) ⇄ CaO(s) + CO_2(g)} \)
Write the equilibrium constant expression and explain why solids are omitted.
▶️ Answer / Explanation
Step 1: Identify the phases: \( \mathrm{CaCO_3} \) and \( \mathrm{CaO} \) are solids, \( \mathrm{CO_2} \) is a gas.
Step 2: Write \( \mathrm{K_p} \) expression, including only the gaseous species:
\( \mathrm{K_p = P_{CO_2}} \)
Step 3: The solids have constant densities, so their “concentrations” do not change — they are omitted from the equilibrium expression.
Final Answer: \( \mathrm{K_p = P_{CO_2}} \); solids \( \mathrm{CaCO_3} \) and \( \mathrm{CaO} \) are excluded.
Example :
Reaction: \( \mathrm{H_2O(l) ⇄ H^+(aq) + OH^-(aq)} \)
Why is \( \mathrm{[H_2O]} \) not included in the expression for the equilibrium constant \( \mathrm{K_w} \)?
▶️ Answer / Explanation
Step 1: The equilibrium constant expression is:
\( \mathrm{K = \dfrac{[H^+][OH^-]}{[H_2O]}} \)
Step 2: The concentration of liquid water is essentially constant (about \( \mathrm{55.5\ mol/L} \) in pure water).
Step 3: Since \( \mathrm{[H_2O]} \) does not vary significantly, it is incorporated into the constant:
\( \mathrm{K_w = [H^+][OH^-]} \)
Final Answer: \( \mathrm{[H_2O]} \) is omitted because it remains constant — it does not affect equilibrium position.
