AP Chemistry 7.5 Magnitude of the Equilibrium Constant Study Notes - New Syllabus Effective fall 2024
AP Chemistry 7.5 Magnitude of the Equilibrium Constant Study Notes- New syllabus
AP Chemistry 7.5 Magnitude of the Equilibrium Constant Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship between very large or very small values of K and the relative concentrations of chemical species at equilibrium.
Key Concepts:
- Relationship Between the Magnitude of K and Equilibrium Composition
Relationship Between the Magnitude of K and Equilibrium Composition
The equilibrium constant (\( \mathrm{K} \)) provides a quantitative measure of how far a chemical reaction proceeds before reaching equilibrium. The magnitude of K indicates whether the equilibrium mixture contains mostly reactants, mostly products, or significant amounts of both.
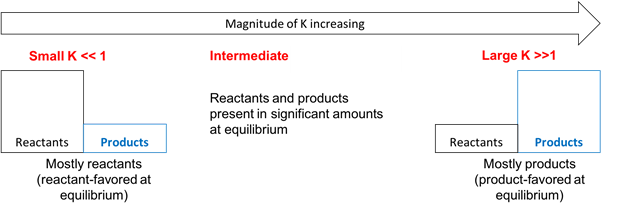
Key Idea:
- If \( \mathrm{K} \) is very large (\( \mathrm{K \gg 1} \)), the reaction strongly favors products at equilibrium — the reaction proceeds nearly to completion.
- If \( \mathrm{K} \) is very small (\( \mathrm{K \ll 1} \)), the reaction favors reactants — very little product is formed at equilibrium.
- If \( \mathrm{K} \) is around 1, both reactants and products are present in comparable amounts.
Relationship Summary:
| Magnitude of \( \mathrm{K} \) | Equilibrium Position | Mixture Composition | Reaction Direction Favored |
|---|---|---|---|
| \( \mathrm{K \gg 1} \) | Far to the right | Mostly products | Forward (→) |
| \( \mathrm{K \approx 1} \) | Intermediate | Comparable reactants and products | Neither strongly favored |
| \( \mathrm{K \ll 1} \) | Far to the left | Mostly reactants | Reverse (←) |
Interpretation of Extreme Values:
- \( \mathrm{K > 10^3} \): Reaction is nearly complete — products dominate.
- \( \mathrm{K < 10^{-3}} \): Hardly any reaction occurs — reactants dominate.
- Intermediate \( \mathrm{K} \): Significant amounts of both reactants and products coexist.
Summary:
- \( \mathrm{K} \) indicates the extent of reaction, not its rate.
- Large \( \mathrm{K} \) → products dominate (reaction “goes to completion”).
- Small \( \mathrm{K} \) → reactants dominate (reaction barely proceeds).
- Intermediate \( \mathrm{K} \) → significant quantities of both reactants and products at equilibrium.
Example
Reaction: \( \mathrm{2H_2(g) + O_2(g) ⇄ 2H_2O(g)} \)
\( \mathrm{K_c = 2.4 \times 10^{47}} \) at 25°C
What does this large \( \mathrm{K_c} \) indicate about the equilibrium mixture?
▶️ Answer / Explanation
Step 1: \( \mathrm{K_c} \) is extremely large, meaning the numerator (products) greatly outweighs the denominator (reactants).
Step 2: The reaction proceeds nearly to completion — almost all \( \mathrm{H_2} \) and \( \mathrm{O_2} \) are converted into \( \mathrm{H_2O} \).
Final Answer: The equilibrium mixture contains mostly \( \mathrm{H_2O} \) vapor — products are overwhelmingly favored.
Example
Reaction: \( \mathrm{N_2(g) + O_2(g) ⇄ 2NO(g)} \)
\( \mathrm{K_p = 4.0 \times 10^{-31}} \) at 25°C
What does this small \( \mathrm{K_p} \) value imply about the equilibrium state?
▶️ Answer / Explanation
Step 1: The very small \( \mathrm{K_p} \) means that the denominator (reactants) dominates in the expression.
Step 2: Only an extremely small amount of \( \mathrm{NO} \) forms — equilibrium lies far to the left.
Step 3: The reaction is thermodynamically unfavorable under these conditions.
Final Answer: The equilibrium mixture contains almost entirely \( \mathrm{N_2} \) and \( \mathrm{O_2} \); product concentration is negligible.
