AP Chemistry 7.6 Properties of the Equilibrium Constant Study Notes - New Syllabus Effective fall 2024
AP Chemistry 7.6 Properties of the Equilibrium Constant Study Notes- New syllabus
AP Chemistry 7.6 Properties of the Equilibrium Constant Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Represent a multistep process with an overall equilibrium expression, using the constituent K expressions for each individual reaction.
Key Concepts:
- When a Reaction Is Reversed, K Is Inverted
- When Stoichiometric Coefficients Are Multiplied, K Is Raised to a Power
- When Reactions Are Added, K Values Are Multiplied
- Algebraic Manipulations of K Also Apply to Q
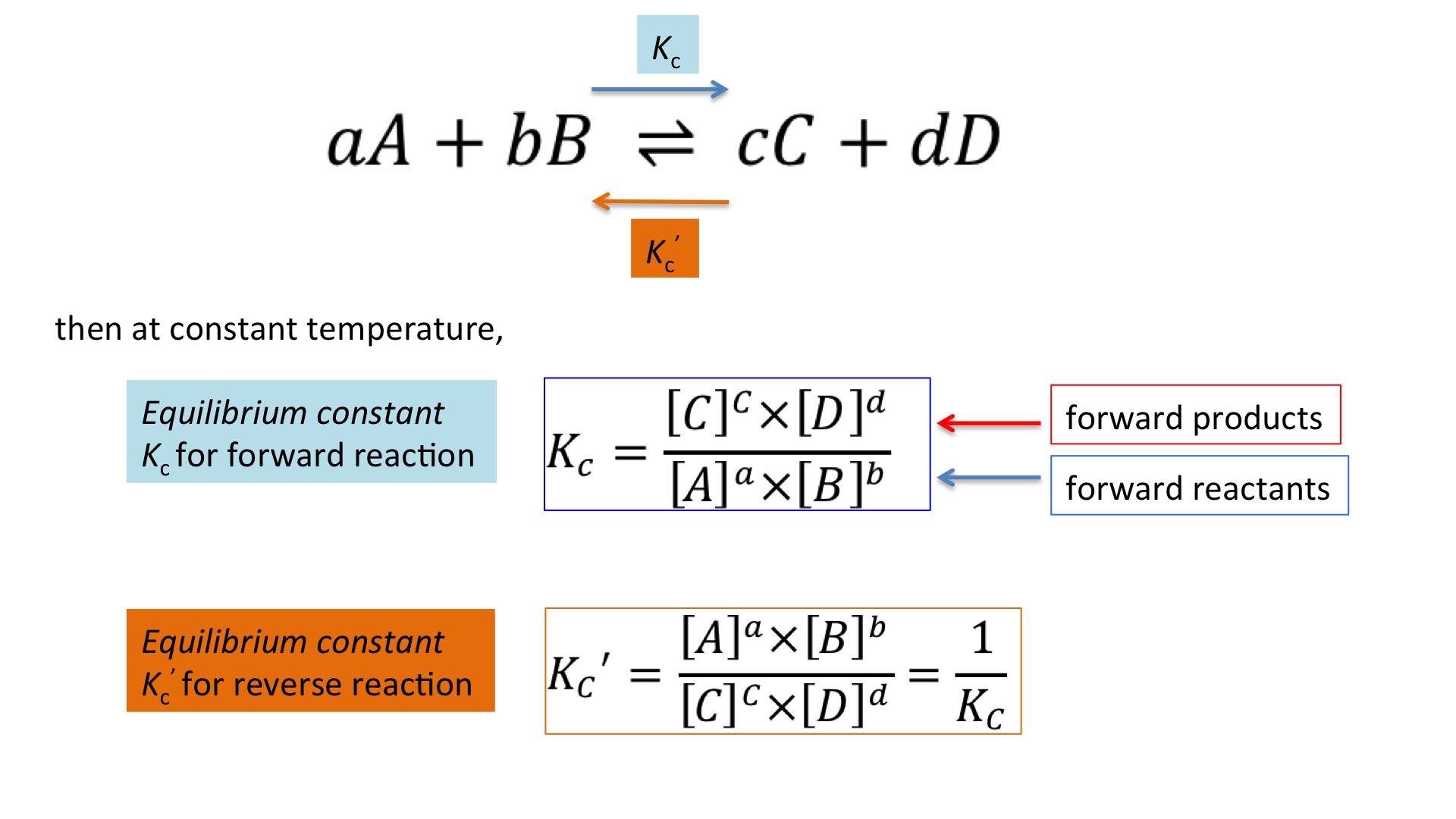
When a Reaction Is Reversed, K Is Inverted
Reversing a chemical equation swaps the roles of reactants and products. Because the equilibrium expression is inverted, the new equilibrium constant becomes the reciprocal of the original \( \mathrm{K} \).
Mathematically:
If \( \mathrm{A ⇄ B} \) has \( \mathrm{K_1 = \dfrac{[B]}{[A]}} \), then reversing it to \( \mathrm{B ⇄ A} \) gives \( \mathrm{K_2 = \dfrac{1}{K_1}} \).
Key Idea: Reversing the direction of a reaction inverts \( \mathrm{K} \).
Example :
Reaction 1: \( \mathrm{N_2O_4(g) ⇄ 2NO_2(g)} \), \( \mathrm{K_c = 0.212} \)
Reversed Reaction: \( \mathrm{2NO_2(g) ⇄ N_2O_4(g)} \) Find new K.
▶️ Answer / Explanation
When reversed, \( \mathrm{K’_c = \dfrac{1}{K_c} = \dfrac{1}{0.212} = 4.72} \).
Final Answer: \( \mathrm{K’_c = 4.72} \)
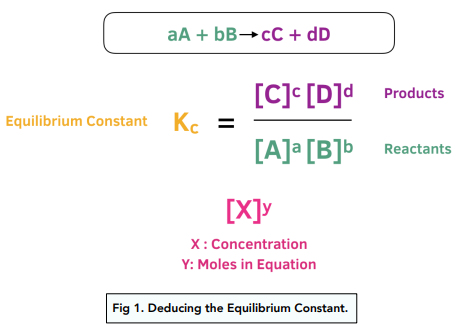
When Stoichiometric Coefficients Are Multiplied, K Is Raised to a Power
If the coefficients in a balanced chemical equation are multiplied by a constant factor \( \mathrm{c} \), then the equilibrium constant \( \mathrm{K} \) is raised to the same power \( \mathrm{c} \).
Mathematically:
For \( \mathrm{aA ⇄ bB} \), \( \mathrm{K = \dfrac{[B]^b}{[A]^a}} \)
If coefficients are multiplied by \( \mathrm{c} \), the new constant becomes \( \mathrm{K’ = (K)^c} \).
Example :
Reaction: \( \mathrm{N_2O_4(g) ⇄ 2NO_2(g)} \), \( \mathrm{K_c = 0.212} \)
Modified Reaction: \( \mathrm{2N_2O_4(g) ⇄ 4NO_2(g)} \) , Find new K.
▶️ Answer / Explanation
All coefficients are multiplied by 2, so \( \mathrm{K’_c = (K_c)^2 = (0.212)^2 = 0.0449} \).
Final Answer: \( \mathrm{K’_c = 0.0449} \)
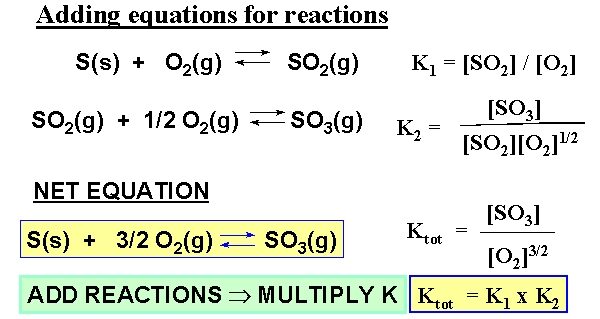
When Reactions Are Added, K Values Are Multiplied
If two (or more) reactions are added together to form an overall reaction, the equilibrium constant for the overall process is the product of the equilibrium constants for the individual steps.
Mathematically:
If \( \mathrm{A ⇄ B} \) has \( \mathrm{K_1} \) and \( \mathrm{B ⇄ C} \) has \( \mathrm{K_2} \),
then \( \mathrm{A ⇄ C} \) has \( \mathrm{K_3 = K_1 \times K_2} \).
Example :
Step 1: \( \mathrm{C ⇄ D} \), \( \mathrm{K_1 = 3.0} \)
Step 2: \( \mathrm{D ⇄ E} \), \( \mathrm{K_2 = 2.5} \)
Overall Reaction: \( \mathrm{C ⇄ E} \) Find new K.
▶️ Answer / Explanation
Step 1: Combine reactions → \( \mathrm{C ⇄ E} \).
Step 2: Multiply constants → \( \mathrm{K_{overall} = K_1 \times K_2 = 3.0 \times 2.5 = 7.5} \).
Final Answer: \( \mathrm{K_{overall} = 7.5} \)
Algebraic Manipulations of K Also Apply to Q
The reaction quotient (Q) has the same mathematical form as the equilibrium constant (K). Therefore, any algebraic manipulation that applies to \( \mathrm{K} \) — such as inversion, exponentiation, or multiplication — also applies to \( \mathrm{Q} \).
- If a reaction is reversed, \( \mathrm{Q’} = \dfrac{1}{Q} \)
- If coefficients are multiplied by \( \mathrm{c} \), \( \mathrm{Q’} = (Q)^c \)
- If reactions are added, \( \mathrm{Q_{overall} = Q_1 \times Q_2} \)
Summary Table:
| Reaction Manipulation | Effect on \( \mathrm{K} \) | Effect on \( \mathrm{Q} \) |
|---|---|---|
| Reaction Reversed | \( \mathrm{K’ = 1/K} \) | \( \mathrm{Q’ = 1/Q} \) |
| Coefficients × c | \( \mathrm{K’ = K^c} \) | \( \mathrm{Q’ = Q^c} \) |
| Reactions Added | \( \mathrm{K_{overall} = K_1 \times K_2} \) | \( \mathrm{Q_{overall} = Q_1 \times Q_2} \) |
Example :
Reaction: \( \mathrm{CO(g) + \tfrac{1}{2}O_2(g) ⇄ CO_2(g)} \)
Expression: \( \mathrm{Q = \dfrac{[CO_2]}{[CO][O_2]^{1/2}}} \)
▶️ Answer / Explanation
If this reaction is doubled:
\( \mathrm{2CO(g) + O_2(g) ⇄ 2CO_2(g)} \)
Then \( \mathrm{Q’ = (Q)^2} \), just as \( \mathrm{K’} = (K)^2} \).
Final Idea: The same mathematical relationships used to modify \( \mathrm{K} \) also apply to \( \mathrm{Q} \).
