AP Chemistry 7.7 Calculating Equilibrium Concentrations Study Notes - New Syllabus Effective fall 2024
AP Chemistry 7.7 Calculating Equilibrium Concentrations Study Notes- New syllabus
AP Chemistry 7.7 Calculating Equilibrium Concentrations Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Identify the concentrations or partial pressures of chemical species at equilibrium based on the initial conditions and the equilibrium constant.
Key Concepts:
- Calculating Equilibrium Concentrations
- Representations of Equilibrium
Predicting Equilibrium Concentrations or Pressures
Given a balanced reaction, initial concentrations (or partial pressures), and the equilibrium constant (\( \mathrm{K_c} \) or \( \mathrm{K_p} \)), we can determine the equilibrium composition of a system using the ICE method (Initial, Change, Equilibrium).
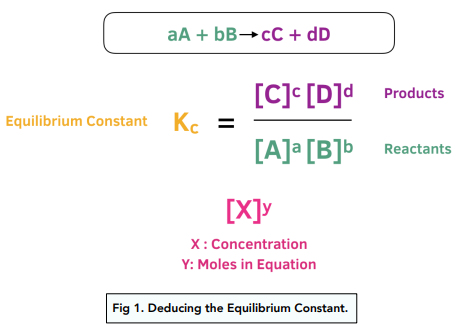
- At equilibrium, concentrations satisfy the equilibrium constant expression.
- Starting from known initial quantities, the changes during the reaction are represented by a variable \( \mathrm{x} \).
- By substituting equilibrium expressions into the equilibrium constant equation, we can solve for \( \mathrm{x} \) and determine all equilibrium concentrations.
Mathematical Setup (ICE Table):
For a general reaction:
\( \mathrm{aA + bB ⇄ cC + dD} \)
| Species | Initial (M) | Change (M) | Equilibrium (M) |
|---|---|---|---|
| A | \( \mathrm{[A]_0} \) | \( \mathrm{-a x} \) | \( \mathrm{[A]_0 – a x} \) |
| B | \( \mathrm{[B]_0} \) | \( \mathrm{-b x} \) | \( \mathrm{[B]_0 – b x} \) |
| C | 0 | \( \mathrm{+c x} \) | \( \mathrm{c x} \) |
| D | 0 | \( \mathrm{+d x} \) | \( \mathrm{d x} \) |
Substitute these equilibrium concentrations into the equilibrium expression to solve for \( \mathrm{x} \).
Example :
Reaction: \( \mathrm{H_2(g) + I_2(g) ⇄ 2HI(g)} \)
Given: \( \mathrm{[H_2]_0 = [I_2]_0 = 1.00\ M, [HI]_0 = 0.00\ M, K_c = 49.0} \)
Calculate the equilibrium concentrations of all species.
▶️ Answer / Explanation
Step 1: Let \( \mathrm{x} \) be the change in \( \mathrm{H_2} \) and \( \mathrm{I_2} \) at equilibrium.
\( \mathrm{[H_2] = [I_2] = 1.00 – x,\ [HI] = 2x} \)
Step 2: Substitute into equilibrium expression:
\( \mathrm{K_c = \dfrac{[HI]^2}{[H_2][I_2]} = 49.0 = \dfrac{(2x)^2}{(1.00 – x)^2}} \)
Step 3: Solve for \( \mathrm{x} \):
\( \mathrm{7 = \dfrac{2x}{1.00 – x}} \Rightarrow 7(1 – x) = 2x \Rightarrow 7 – 7x = 2x \Rightarrow x = 0.778} \)
Step 4: Find equilibrium concentrations:
- \( \mathrm{[H_2] = [I_2] = 1.00 – 0.778 = 0.222\ M} \)
- \( \mathrm{[HI] = 2(0.778) = 1.56\ M} \)
Final Answer: \( \mathrm{[H_2]_{eq} = [I_2]_{eq} = 0.222\ M} \), \( \mathrm{[HI]_{eq} = 1.56\ M} \).
Relationship Between Q, K, and Reaction Direction
The reaction quotient (Q) compares the current ratio of products to reactants to the equilibrium ratio (\( \mathrm{K} \)). By comparing \( \mathrm{Q} \) and \( \mathrm{K} \), we can determine which direction the reaction must proceed to reach equilibrium.
Key Relationship:
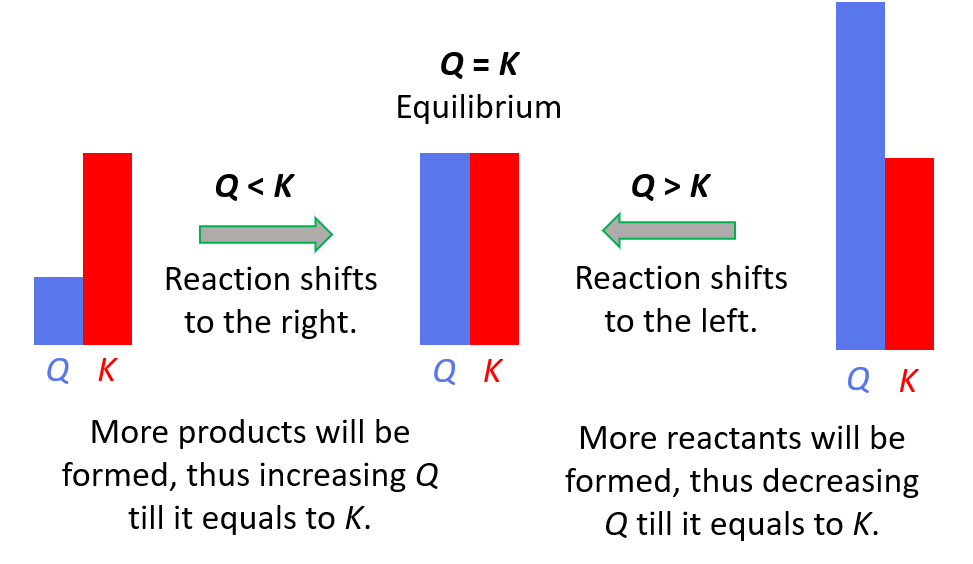
- \( \mathrm{Q < K} \) → Reaction shifts forward (toward products).
- \( \mathrm{Q > K} \) → Reaction shifts reverse (toward reactants).
- \( \mathrm{Q = K} \) → System is at dynamic equilibrium.
Mathematical Expression:
For a reaction \( \mathrm{aA + bB ⇄ cC + dD} \):
\( \mathrm{Q_c = \dfrac{[C]^c [D]^d}{[A]^a [B]^b}} \)
The same expression applies for \( \mathrm{K_c} \); the only difference is whether the system is at equilibrium.
Summary Table:
| Condition | Comparison | Shift Direction | Effect |
|---|---|---|---|
| Reactants too high | \( \mathrm{Q < K} \) | Forward (→) | Products increase |
| Products too high | \( \mathrm{Q > K} \) | Reverse (←) | Reactants increase |
| At equilibrium | \( \mathrm{Q = K} \) | No net shift | Dynamic equilibrium maintained |
Key Idea: The relationship between \( \mathrm{Q} \) and \( \mathrm{K} \) determines which direction the system shifts to reach equilibrium. Once equilibrium is reached, all forward and reverse reaction rates are equal, and concentrations remain constant.
Example :
Reaction: \( \mathrm{N_2(g) + 3H_2(g) ⇄ 2NH_3(g)} \)
Given: \( \mathrm{K_c = 6.0 \times 10^5} \) at 400 K
Concentrations: \( \mathrm{[N_2] = 0.20\ M}, \mathrm{[H_2] = 0.60\ M}, \mathrm{[NH_3] = 0.10\ M} \)
Will the reaction shift forward or backward to reach equilibrium?
▶️ Answer / Explanation
Step 1: Calculate \( \mathrm{Q_c} \):
\( \mathrm{Q_c = \dfrac{[NH_3]^2}{[N_2][H_2]^3} = \dfrac{(0.10)^2}{(0.20)(0.60)^3} = 0.10/0.0432 = 2.31} \)
Step 2: Compare \( \mathrm{Q_c} \) to \( \mathrm{K_c} \):
\( \mathrm{Q_c = 2.31} \) and \( \mathrm{K_c = 6.0 \times 10^5} \Rightarrow Q_c < K_c \).
Step 3: Interpretation:
Because \( \mathrm{Q_c < K_c} \), the reaction will shift forward (toward products) until equilibrium is achieved.
Final Answer: The reaction proceeds forward, forming more \( \mathrm{NH_3} \).
