AP Chemistry 7.8 Representations of Equilibrium Study Notes - New Syllabus Effective fall 2024
AP Chemistry 7.8 Representations of Equilibrium Study Notes New syllabus
AP Chemistry 7.8 Representations of Equilibrium Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Represent a system undergoing a reversible reaction with a particulate model.
Key Concepts:
- Particulate Representation of Reversible Reactions and Equilibrium
Particulate Representation of Reversible Reactions and Equilibrium
A particulate model visually represents a chemical system at the microscopic level. It shows the relative number of particles of reactants and products before the reaction, during the reaction, and at equilibrium. At equilibrium, the forward and reverse reactions occur at equal rates, so the number of reactant and product particles remains constant over time.
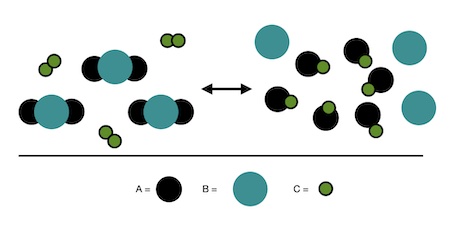
Key Idea:
- At equilibrium, reactants and products are both present — neither side is fully consumed.
- The relative number of product and reactant particles depends on the value of the equilibrium constant (\( \mathrm{K} \)).
- Particulate models can visually demonstrate whether \( \mathrm{K} \) is large, small, or approximately equal to 1.
Relationship Between Particle Ratios and \( \mathrm{K} \):
| Relative Particle Distribution | Value of \( \mathrm{K} \) | Interpretation |
|---|---|---|
| Mostly product particles | \( \mathrm{K \gg 1} \) | Reaction lies far to the right — product-favored |
| Comparable reactants and products | \( \mathrm{K \approx 1} \) | Neither side strongly favored |
| Mostly reactant particles | \( \mathrm{K \ll 1} \) | Reaction lies far to the left — reactant-favored |
Microscopic Description of Equilibrium:
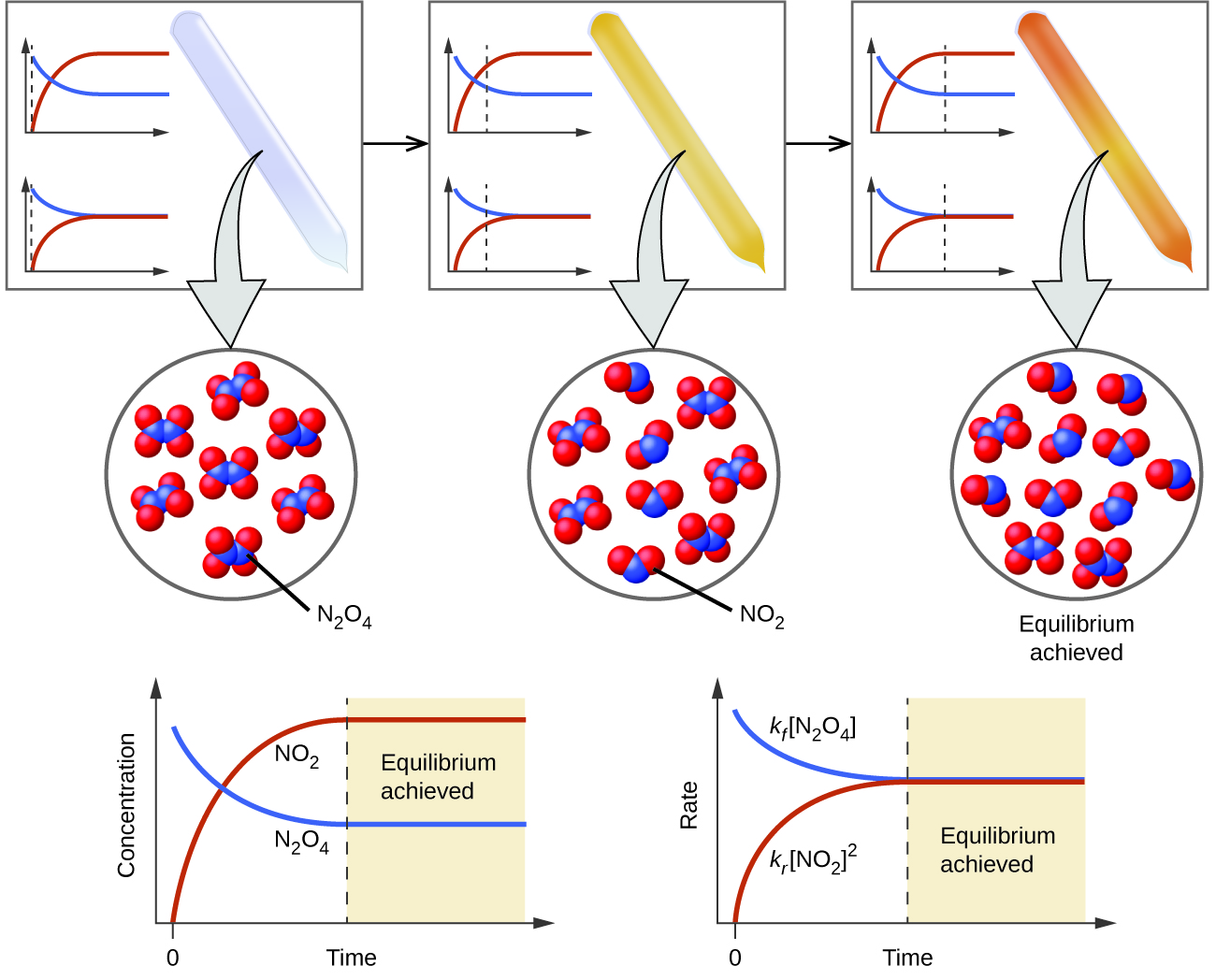
- Before equilibrium: forward reaction rate \( \mathrm{>} \) reverse reaction rate → product particles increase.
- At equilibrium: forward rate = reverse rate → number of reactant and product particles remains constant.
- Even though macroscopic concentrations appear constant, particles continue to react — equilibrium is dynamic.
Example :
Reaction: \( \mathrm{A_2(g) ⇄ 2A(g)} \)
Scenario: Three containers at equilibrium show different numbers of particles:
- Container X: 2 \( \mathrm{A_2} \) molecules, 10 \( \mathrm{A} \) atoms
- Container Y: 6 \( \mathrm{A_2} \) molecules, 6 \( \mathrm{A} \) atoms
- Container Z: 10 \( \mathrm{A_2} \) molecules, 2 \( \mathrm{A} \) atoms
Rank the values of \( \mathrm{K} \) for X, Y, and Z.
▶️ Answer / Explanation
Step 1: The reaction \( \mathrm{A_2 ⇄ 2A} \) has \( \mathrm{K = \dfrac{[A]^2}{[A_2]}} \).
Step 2: Container X has mostly products → \( \mathrm{K} \) is large.
Container Z has mostly reactants → \( \mathrm{K} \) is small.
Step 3: Rank them: \( \mathrm{K_X > K_Y > K_Z} \).
Final Answer: X (product-favored) → largest \( \mathrm{K} \); Z (reactant-favored) → smallest \( \mathrm{K} \).
Example :
Reaction: \( \mathrm{N_2O_4(g) ⇄ 2NO_2(g)} \)
The diagram shows constant numbers of \( \mathrm{N_2O_4} \) and \( \mathrm{NO_2} \) particles, but both collisions (decomposition and recombination) continue. What conclusion can you draw?
▶️ Answer / Explanation
Step 1: The constant particle numbers indicate no net change in macroscopic concentrations.
Step 2: Continuous collisions indicate the reaction is ongoing in both directions.
Step 3: Therefore, the system is at dynamic equilibrium — forward and reverse reaction rates are equal.
Final Answer: The system is at equilibrium; \( \mathrm{K} \) reflects the ratio \( \mathrm{[NO_2]^2/[N_2O_4]} \), which remains constant.
