AP Chemistry 7.9 Introduction to Le Chatelier’s Principle Study Notes - New Syllabus Effective fall 2024
AP Chemistry 7.9 Introduction to Le Chatelier’s Principle Study Notes- New syllabus
AP Chemistry 7.9 Introduction to Le Chatelier’s Principle Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Identify the response of a system at equilibrium to an external stress, using Le Châtelier’s principle.
Key Concepts:
- Le Châtelier’s Principle
- Predicting Observable Effects (pH, Color, Temperature)
Le Châtelier’s Principle
Le Châtelier’s Principle states that when a system at equilibrium is disturbed by a change in conditions (such as concentration, pressure, or temperature), the system shifts in the direction that counteracts the disturbance and restores a new equilibrium state.
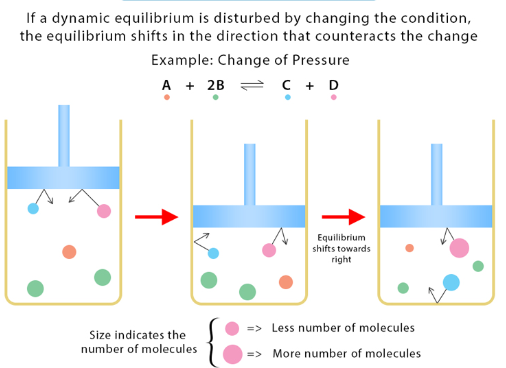
Key Idea:
- Equilibrium shifts occur to reduce the applied stress.
- The equilibrium constant \( \mathrm{K} \) remains unchanged by concentration or pressure changes — only temperature affects \( \mathrm{K} \).
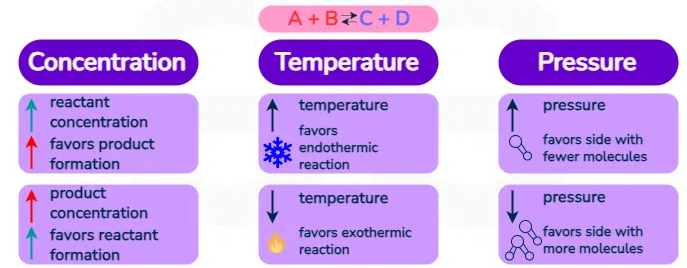
Common Stresses and Their Effects:
| Type of Change | Effect on System | Shift Direction |
|---|---|---|
| Add reactant | System consumes added species | Toward products (→) |
| Remove reactant | System replaces removed species | Toward reactants (←) |
| Increase pressure (gas system) | Favors fewer gas moles | Toward fewer moles of gas |
| Decrease pressure | Favors more gas moles | Toward more moles of gas |
| Increase temperature (endothermic) | Heat acts as a reactant | Toward products (→) |
| Increase temperature (exothermic) | Heat acts as a product | Toward reactants (←) |
Example : Effect of Concentration Change
Reaction: \( \mathrm{N_2(g) + 3H_2(g) ⇄ 2NH_3(g)} \) (exothermic)
Predict the effect of adding more \( \mathrm{N_2} \) to the system at equilibrium.
▶️ Answer / Explanation
Step 1: Adding \( \mathrm{N_2} \) increases the reactant concentration → system temporarily has \( \mathrm{Q < K} \).
Step 2: To re-establish equilibrium, the system shifts right to consume added \( \mathrm{N_2} \).
Final Answer: The reaction shifts → right (toward \( \mathrm{NH_3} \)), producing more ammonia.
Example : Effect of Pressure Change
Reaction: \( \mathrm{2SO_2(g) + O_2(g) ⇄ 2SO_3(g)} \)
Predict how increasing pressure affects the position of equilibrium.
▶️ Answer / Explanation
Step 1: Left side = 3 moles gas; right side = 2 moles gas.
Step 2: Increasing pressure favors the side with fewer gas moles.
Final Answer: The equilibrium shifts → right (toward \( \mathrm{SO_3} \)).
Example : Effect of Temperature Change
Reaction: \( \mathrm{2NO_2(g) ⇄ N_2O_4(g)} \) ΔH = −58 kJ (exothermic)
Predict the effect of increasing temperature.
▶️ Answer / Explanation
Step 1: Heat is a product → \( \mathrm{2NO_2 ⇄ N_2O_4 + heat} \).
Step 2: Increasing T adds heat → system shifts left (endothermic direction) to absorb it.
Step 3: \( \mathrm{K} \) decreases (since exothermic).
Final Answer: Reaction shifts ← left; \( \mathrm{NO_2} \) concentration increases; color becomes darker brown.
Predicting Observable Effects (pH, Color, Temperature)
Le Châtelier’s principle can also be used to predict measurable changes in observable properties such as color, pH, or temperature when a system is stressed.
Key Observable Properties Affected:
- pH changes: adding acid/base shifts acid–base equilibria.
- Color changes: equilibrium involving colored ions (e.g., \( \mathrm{[Co(H_2O)_6]^{2+}} \) pink ⇄ \( \mathrm{[CoCl_4]^{2−}} \) blue).
- Temperature changes: for exothermic reactions, increasing T makes the mixture darker (because the endothermic direction is favored).
Core Insights:
- Le Châtelier’s principle explains both qualitative and observable shifts in equilibrium systems.
- Only temperature changes affect \( \mathrm{K} \); concentration or pressure changes temporarily change \( \mathrm{Q} \) until equilibrium re-establishes.
- Observable quantities (color, pH, gas volume, temperature) provide experimental evidence of equilibrium shift direction.
Example :
Reaction: \( \mathrm{[Co(H_2O)_6]^{2+}(aq)\ (pink) + 4Cl^-(aq) ⇄ [CoCl_4]^{2−}(aq)\ (blue) + 6H_2O(l)} \)
Predict the effect of adding more \( \mathrm{Cl^-} \) or increasing T.
▶️ Answer / Explanation
Adding Cl⁻: shifts right → blue color intensifies (product favored).
Increasing Temperature: reaction is endothermic → shifts right → blue color increases further.
Final Observation: More Cl⁻ or heat = blue; cooling or removing Cl⁻ = pink.
Example :
Reaction: \( \mathrm{HCO_3^- (aq) + H_2O(l) ⇄ H_3O^+ (aq) + CO_3^{2−}(aq)} \)
What happens to pH when acid or base is added?
▶️ Answer / Explanation
Add acid (\( \mathrm{H_3O^+} \)): shifts left → decreases \( \mathrm{CO_3^{2−}} \); pH drops slightly but buffer resists change.
Add base (\( \mathrm{OH^-} \)): consumes \( \mathrm{H_3O^+} \), shifts right → increases \( \mathrm{CO_3^{2−}} \); pH rises slightly.
Final Result: The bicarbonate system resists large pH changes — behavior of a buffer.
