AP Chemistry 8.1 Introduction to Acids and Bases Study Notes - New Syllabus Effective fall 2024
AP Chemistry 8.1 Introduction to Acids and Bases Study Notes- New syllabus
AP Chemistry 8.1 Introduction to Acids and Bases Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Calculate the values of pH and pOH, based on Kw and the concentration of all species present in a neutral solution of water.
Key Concepts:
- Definition of pH and pOH
- Autoionization of Water and the Ionic Product Constant \( \mathrm{K_w} \)
- Relationship Between pH, pOH, and \( \mathrm{pK_w} \)
- Temperature Dependence of \( \mathrm{K_w} \) and Its Effect on Neutral pH
Definition of pH and pOH
The acidity or basicity of an aqueous solution is expressed in terms of the concentrations of hydronium ions (\( \mathrm{H_3O^+} \)) and hydroxide ions (\( \mathrm{OH^-} \)). These quantities are conveniently represented on logarithmic scales called pH and pOH.
Key Formulas
\( \mathrm{pH = -\log [H_3O^+]} \)
\( \mathrm{pOH = -\log [OH^-]} \)
- \( \mathrm{[H_3O^+]} \): molar concentration of hydronium ion (mol/L)
- \( \mathrm{[OH^-]} \): molar concentration of hydroxide ion (mol/L)
Terminology Note:
- The symbols \( \mathrm{H^+(aq)} \) and \( \mathrm{H_3O^+(aq)} \) are often used interchangeably to represent the same species.
- \( \mathrm{H_3O^+(aq)} \) or “hydronium ion” is preferred for accuracy, but \( \mathrm{H^+(aq)} \) is acceptable on the AP Exam.
Interpretation:
- Lower pH → higher \( \mathrm{[H_3O^+]} \) → more acidic solution.
- Higher pH → lower \( \mathrm{[H_3O^+]} \) → more basic solution.
- pOH behaves inversely — higher pOH indicates more acidic conditions.
Example:
A solution has \( \mathrm{[H_3O^+] = 1.0 \times 10^{-3}\ M} \). Calculate its pH and pOH (at 25 °C).
▶️ Answer / Explanation
Step 1: Use the definition of pH.
\( \mathrm{pH = -\log(1.0 \times 10^{-3}) = 3.00} \)
Step 2: Use the relationship \( \mathrm{pH + pOH = 14.00} \).
\( \mathrm{pOH = 14.00 – 3.00 = 11.00} \)
Final Answer: pH = 3.00 → acidic solution pOH = 11.00
Autoionization of Water and the Ionic Product Constant \( \mathrm{K_w} \)
Even pure water contains a very small number of ions due to a process called autoionization (or self-ionization). In this process, two water molecules react with each other — one acts as a Brønsted-Lowry acid and the other as a base — producing hydronium ions (\( \mathrm{H_3O^+} \)) and hydroxide ions (\( \mathrm{OH^-} \)).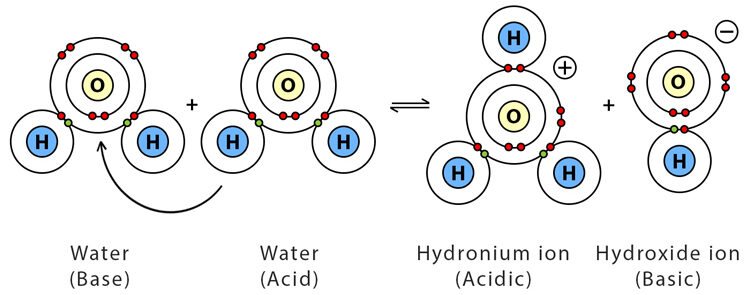
\( \mathrm{2H_2O(l) \rightleftharpoons H_3O^+(aq) + OH^-(aq)} \)
Equilibrium Expression for Autoionization
\( \mathrm{K_w = [H_3O^+][OH^-]} \)
- \( \mathrm{K_w} \): ionic product constant of water (also called water dissociation constant)
- \( \mathrm{[H_3O^+]} \): molar concentration of hydronium ions (mol/L)
- \( \mathrm{[OH^-]} \): molar concentration of hydroxide ions (mol/L)
At 25 °C (298 K):
\( \mathrm{K_w = 1.0 \times 10^{-14}} \)
Interpretation: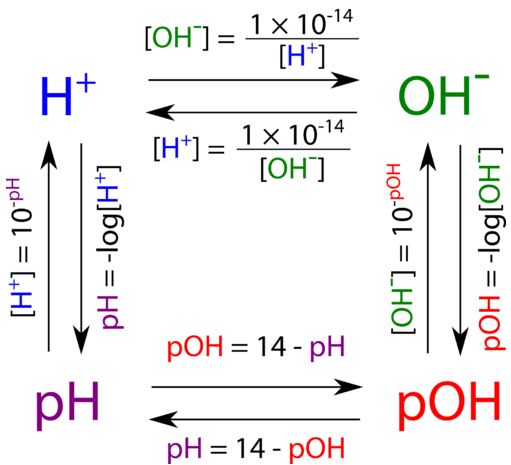
- In pure water at 25 °C, \( \mathrm{[H_3O^+] = [OH^-] = 1.0 \times 10^{-7}\ M} \).
- The very small value of \( \mathrm{K_w} \) indicates that water ionizes only slightly.
- As temperature increases, \( \mathrm{K_w} \) increases (autoionization is endothermic).
Relation Between pH, pOH, and \( \mathrm{K_w} \)
Since \( \mathrm{K_w = [H_3O^+][OH^-]} \), taking the negative logarithm gives:
\( \mathrm{pK_w = pH + pOH} \)
At 25 °C, \( \mathrm{pK_w = 14.00} \).
Example:
What are the concentrations of \( \mathrm{H_3O^+} \) and \( \mathrm{OH^-} \) in pure water at 25 °C?
▶️ Answer / Explanation
Step 1: Write the expression for \( \mathrm{K_w} \).
\( \mathrm{K_w = [H_3O^+][OH^-]} \)
Step 2: In pure water, \( \mathrm{[H_3O^+] = [OH^-] = x} \).
\( \mathrm{K_w = x^2} \)
Step 3: Substitute \( \mathrm{K_w = 1.0 \times 10^{-14}} \).
\( \mathrm{x = \sqrt{1.0 \times 10^{-14}} = 1.0 \times 10^{-7}\ M} \)
Final Answer: \( \mathrm{[H_3O^+] = [OH^-] = 1.0 \times 10^{-7}\ M} \)
Relationship Between pH, pOH, and \( \mathrm{pK_w} \)
In any aqueous solution, the concentrations of hydronium ions (\( \mathrm{H_3O^+} \)) and hydroxide ions (\( \mathrm{OH^-} \)) are linked through the equilibrium constant for water autoionization, \( \mathrm{K_w} \).
This relationship forms the mathematical foundation of the pH scale and defines the link between acidity and basicity of a solution.
Key Relationships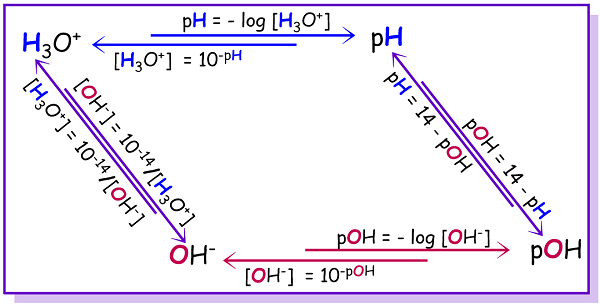
\( \mathrm{K_w = [H_3O^+][OH^-]} \)
Taking the negative logarithm of both sides:
\( \mathrm{-\log K_w = -\log [H_3O^+] – \log [OH^-]} \)
By definition:
- \( \mathrm{pH = -\log [H_3O^+]} \)
- \( \mathrm{pOH = -\log [OH^-]} \)
- \( \mathrm{pK_w = -\log K_w} \)
Thus, the relationship becomes:
\( \mathrm{pH + pOH = pK_w} \)
At 25 °C (298 K):
- \( \mathrm{K_w = 1.0 \times 10^{-14}} \)
- \( \mathrm{pK_w = 14.00} \)
Therefore, under standard temperature conditions:
\( \mathrm{pH + pOH = 14.00} \)
Classification of Solutions (at 25 °C):
| Solution Type | Relationship | pH | pOH |
|---|---|---|---|
| Neutral | \( \mathrm{[H_3O^+] = [OH^-]} \) | 7.00 | 7.00 |
| Acidic | \( \mathrm{[H_3O^+] > [OH^-]} \) | pH < 7 | pOH > 7 |
| Basic | \( \mathrm{[H_3O^+] < [OH^-]} \) | pH > 7 | pOH < 7 |
Interpretation:
- The sum of pH and pOH is constant at a given temperature (14.00 at 25 °C).
- Knowing either pH or pOH allows calculation of the other.
- As pH decreases, pOH increases — maintaining the same \( \mathrm{pK_w} \).
Example:
The pH of an aqueous solution is 4.25 at 25 °C. Calculate the pOH of the solution.
▶️ Answer / Explanation
Step 1: Use the relationship \( \mathrm{pH + pOH = 14.00} \).
Step 2: Substitute the known value.
\( \mathrm{pOH = 14.00 – 4.25 = 9.75} \)
Step 3: Interpretation:
- The solution is acidic because pH < 7.
- Its hydroxide ion concentration is low.
Final Answer: \( \mathrm{pOH = 9.75} \)
Temperature Dependence of \( \mathrm{K_w} \) and Its Effect on Neutral pH
The ionic product of water (\( \mathrm{K_w} \)) is temperature dependent because the autoionization of water is an endothermic process. As temperature increases, water ionizes slightly more, increasing both \( \mathrm{[H_3O^+]} \) and \( \mathrm{[OH^-]} \). This change affects the value of \( \mathrm{K_w} \), and therefore the pH of a neutral solution.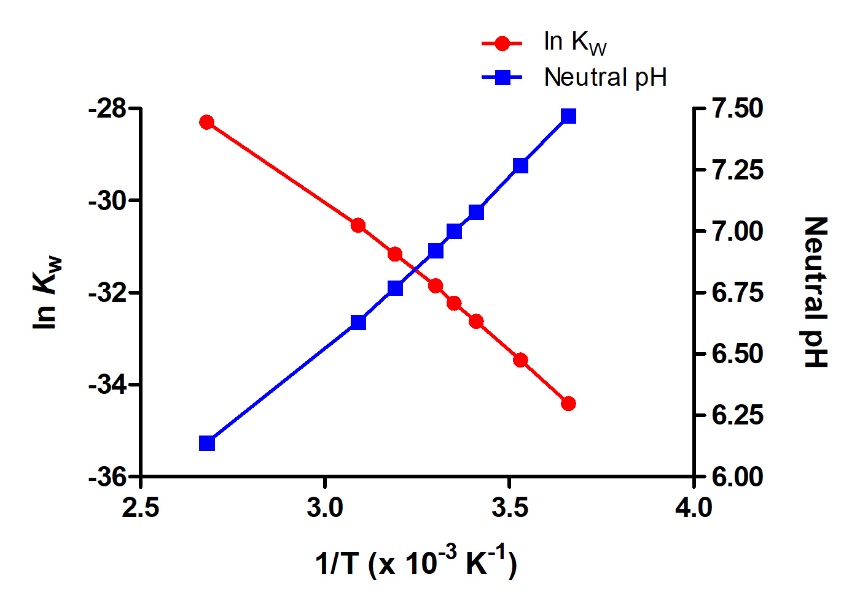
Equilibrium for Autoionization of Water:
\( \mathrm{2H_2O(l) \rightleftharpoons H_3O^+(aq) + OH^-(aq)} \)
Since this reaction absorbs heat (endothermic), an increase in temperature shifts the equilibrium to the right, producing more ions and increasing \( \mathrm{K_w} \).
Temperature Dependence of \( \mathrm{K_w} \):
| Temperature (°C) | \( \mathrm{K_w} \) | \( \mathrm{pK_w} \) | pH of Neutral Water |
|---|---|---|---|
| 0 °C | \( \mathrm{0.11 \times 10^{-14}} \) | 14.04 | 7.02 |
| 25 °C | \( \mathrm{1.0 \times 10^{-14}} \) | 14.00 | 7.00 |
| 50 °C | \( \mathrm{5.5 \times 10^{-14}} \) | 13.26 | 6.63 |
Interpretation:
- As temperature increases → \( \mathrm{K_w} \) increases → \( \mathrm{pK_w} \) decreases.
- Because \( \mathrm{[H_3O^{+}] = [OH^-]} \) in neutral water, both ion concentrations increase equally.
- Thus, neutral water at higher temperatures has pH < 7, even though it is still neutral (equal \( \mathrm{[H_3O^+]} \) and \( \mathrm{[OH^-]} \)).
- At lower temperatures, \( \mathrm{K_w} \) decreases → fewer ions → pH > 7 for neutral water.
Mathematical Relationship:
\( \mathrm{pH_{neutral} = \dfrac{1}{2} pK_w} \)
- At 25 °C → \( \mathrm{pH_{neutral} = 7.00} \)
- At 50 °C → \( \mathrm{pH_{neutral} = 6.63} \)
- At 0 °C → \( \mathrm{pH_{neutral} = 7.02} \)
Example:
Calculate the pH of neutral water at 50 °C if \( \mathrm{K_w = 5.5 \times 10^{-14}} \).
▶️ Answer / Explanation
Step 1: In neutral water, \( \mathrm{[H_3O^+] = [OH^-] = x} \).
Step 2: \( \mathrm{K_w = x^2} \Rightarrow x = \sqrt{5.5 \times 10^{-14}} = 2.35 \times 10^{-7}\ M \)
Step 3: Calculate pH: \( \mathrm{pH = -\log [H_3O^+] = -\log(2.35 \times 10^{-7}) = 6.63} \)
Step 4: Interpretation:
- The pH is < 7, but water is still neutral because \( \mathrm{[H_3O^+] = [OH^-]} \).
- This confirms that “neutral” does not always mean “pH = 7”.
Final Answer: \( \mathrm{pH_{neutral} = 6.63\ at\ 50°C} \)
