Introduction to Acids and Bases
Acids
- All acids start with H
- Covalent bonds bcuz hydrogen bonds with nonmetals
Naming acids
- Binary: hydro___ic acid
- Polyatomic: NO hydro
- -ate = __ic acid
- -ite = __ous acid
Bases
- Ionic bonds because has cation with anion
Naming Bases
- Name first element and end with hydroxide
Models of acids and bases
- At equilibrium:

- There is a competition for H+ between H2O and A-
- The stronger base controls direction → The direction of equilibrium depends on if the acid is weak or strong
- If H2O is a stronger base than A-, (H2O attracts H+ more) → forward reaction favored → most of the acid dissolved will be in the ionized form
- If A- is a much stronger base than H2O → reverse reaction favored → at equilibrium most of the acid will exist as HA
- Monoprotic acids: one acidic hydrogen
- Polyprotic acids: more than one acidic hydrogen which can donate to the solution
- Oxyacids: acidic hydrogen is attached to the oxygen of an ion
- Organic Acids: acids that contain carbon and usually a carboxyl group, generally very weak
Water as an Acid and a Base
- Amphoteric: it can behave either as an acid or a base (ex: water, HSO4-)
- As an acid:
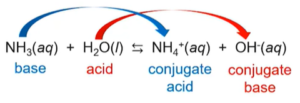
- As a base:
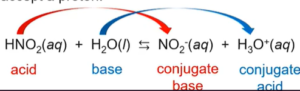
- As an acid:
- Water is amphoteric and autoionizes:

- Ex:
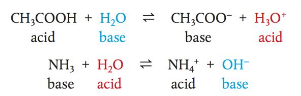
- Ex:
 (on RFS)
(on RFS) - Remember that solids and liquids are not included; temperature dependent
- Neutral solution: [H+] = [OH-]
- Acidic solution: [H+] > [OH-]
- Basic solution: [H+] < [OH-]
The pH Scale
- As pH decreases, [H+] increases exponentially
- The pH changes by 1 for every power of 10 change in [H+]
- Sig Figs & pH: number of sig figs of molarity = number of decimal places in pH
- Ex:
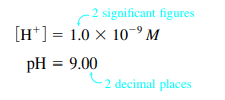
- Ex:
Relationships (All on RFS)
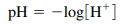
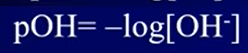
- P = (-) log of …
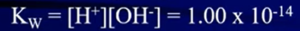
- Ionization increases with increasing temperature (kW will be greater value)

- Subtract 14 from pOH to find pH
 → Given any one of these → can find the other three
→ Given any one of these → can find the other three
Estimating Values When Not Given a Calculator
- Finding the -log of smthn places answer close to exponent
- If first term is exactly 1.0 → -log of it will be the same as exponent
- The greater the first term, the greater the answer is going to fall below the exponent
- If first term is pi, -log of it will fall halfway between the exponent
- Ex:

- Ex:
