AP Chemistry 8.10 Buffer Capacity Study Notes - New Syllabus Effective fall 2024
AP Chemistry 8.10 Buffer Capacity Study Notes- New syllabus
AP Chemistry 8.10 Buffer Capacity Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship between the buffer capacity of a solution and the relative concentrations of the conjugate acid and conjugate base components of the solution.
Key Concepts:
- Effect of Concentration on Buffer Capacity
- Relationship Between Acid/Base Ratio and Buffer Capacity Direction
Effect of Concentration on Buffer Capacity
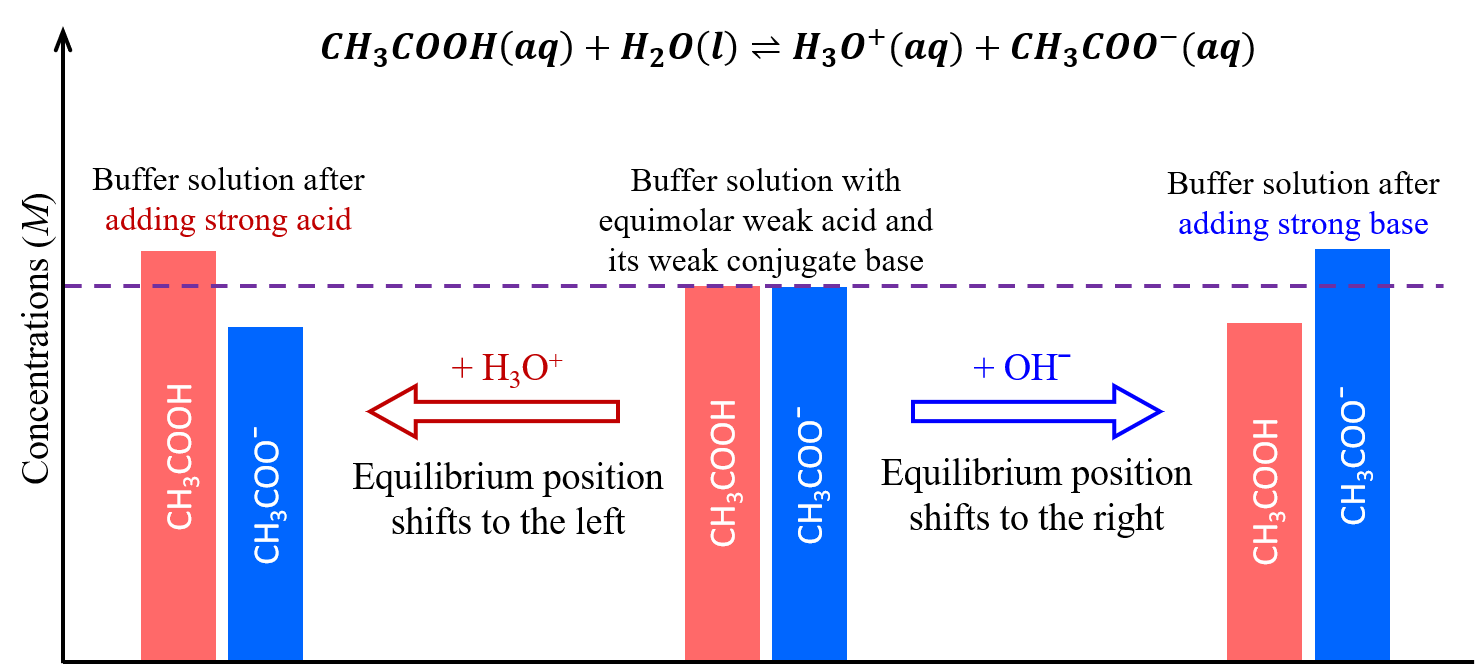
Buffer capacity is the measure of a buffer’s ability to resist changes in pH when acid or base is added. It depends on the absolute concentrations of the acid–base pair, not just their ratio.
Key Idea:
- Increasing the concentrations of both buffer components (\( \mathrm{[HA]} \) and \( \mathrm{[A^-]} \)) by the same factor does not change the pH, because the ratio \( \mathrm{[A^-]/[HA]} \) remains the same in the Henderson–Hasselbalch equation.
- However, a buffer with higher component concentrations can neutralize more acid or base before the pH changes appreciably.
- This means buffer capacity increases even though the buffer pH remains constant.
Mathematical Explanation:
\( \mathrm{pH = pK_a + \log\!\left(\dfrac{[A^-]}{[HA]}\right)} \)
→ If both \( \mathrm{[A^-]} \) and \( \mathrm{[HA]} \) are multiplied by the same factor, the ratio remains constant → pH unchanged.
→ But total moles of \( \mathrm{A^-} \) and \( \mathrm{HA]} \) increase → buffer can absorb more added acid or base.
Example:
Compare the buffer capacities of these two buffers, and state which can better resist pH change.
- Buffer 1: 0.10 M \( \mathrm{CH_3COOH} \) and 0.10 M \( \mathrm{CH_3COONa} \)
- Buffer 2: 1.00 M \( \mathrm{CH_3COOH} \) and 1.00 M \( \mathrm{CH_3COONa} \)
▶️ Answer / Explanation
Step 1: The ratio \( \mathrm{[A^-]/[HA]} = 1 \) for both buffers → same pH.
Step 2: Buffer 2 has 10× more total acid and base molecules → higher capacity to neutralize added acid or base.
Final Answer: Both buffers have the same pH, but Buffer 2 has greater buffer capacity and resists pH change more effectively.
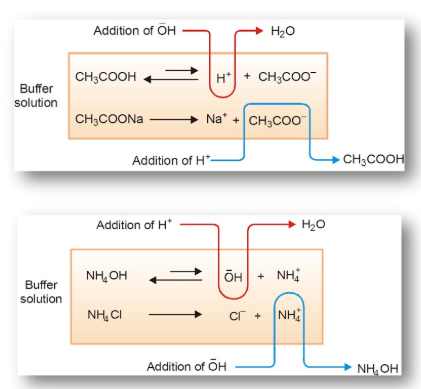
Relationship Between Acid/Base Ratio and Buffer Capacity Direction
The buffer’s ability to neutralize added acid or base depends on the relative amounts of its two components: the weak acid (\( \mathrm{HA} \)) and its conjugate base (\( \mathrm{A^-} \)). The component that is present in greater concentration determines which type of addition the buffer can better resist.
Key Relationships:
- If \( \mathrm{[HA] > [A^-]} \) → buffer contains more acid form → better at neutralizing added base (\( \mathrm{OH^-} \)).
- If \( \mathrm{[A^-] > [HA]} \) → buffer contains more base form → better at neutralizing added acid (\( \mathrm{H_3O^+} \)).
- The total buffer capacity is greatest when \( \mathrm{[A^-] = [HA]} \), i.e., when \( \mathrm{pH = pK_a} \).
Explanation of Buffer Action:
- Added acid reacts with the conjugate base: \( \mathrm{A^- + H_3O^+ \rightarrow HA + H_2O} \)
- Added base reacts with the conjugate acid: \( \mathrm{HA + OH^- \rightarrow A^- + H_2O} \)
The component present in larger amount provides more moles to consume added acid or base, thus offering greater buffering capacity in that direction.
Example:
A buffer is prepared from 0.40 M acetic acid and 0.10 M sodium acetate. For which addition (acid or base) will the buffer have greater capacity?
▶️ Answer / Explanation
Step 1: Compare component concentrations: \( \mathrm{[HA] = 0.40\ M > [A^-] = 0.10\ M} \).
Step 2: More acid present → stronger ability to react with added base (\( \mathrm{OH^-} \)).
Step 3: The buffer can neutralize more base before significant pH change, but it will be less effective against added acid.
Final Answer: This buffer has greater capacity for neutralizing added base.
Example:
A buffer is prepared from 0.10 M acetic acid and 0.40 M sodium acetate. Which addition can it better resist?
▶️ Answer / Explanation
Step 1: \( \mathrm{[A^-] = 0.40\ M > [HA] = 0.10\ M} \)
Step 2: More conjugate base present → stronger ability to react with added acid (\( \mathrm{H_3O^+} \)).
Step 3: The buffer resists decreases in pH more effectively.
Final Answer: This buffer has greater capacity for neutralizing added acid.
