AP Chemistry 8.2 pH and pOH of Strong Acids and Bases Study Notes - New Syllabus Effective fall 2024
AP Chemistry 8.2 pH and pOH of Strong Acids and Bases Study Notes- New syllabus
AP Chemistry 8.2 pH and pOH of Strong Acids and Bases Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Calculate pH and pOH based on concentrations of all species in a solution of a strong acid or a strong base.
Key Concepts:
Ionization of Strong Acids
Dissociation of Strong Bases
Ionization of Strong Acids
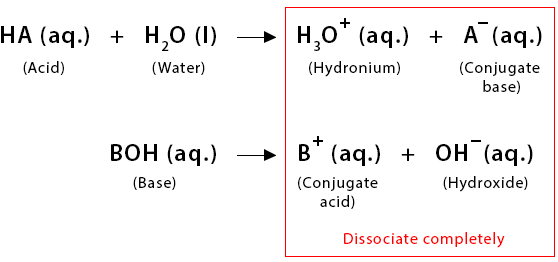
A strong acid completely ionizes in aqueous solution to produce hydronium ions (\( \mathrm{H_3O^+} \)) and its conjugate base. Because the ionization is complete, the concentration of \( \mathrm{H_3O^+} \) equals the initial acid concentration.
Ionization Reaction (General):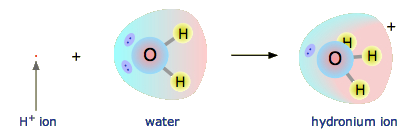
\( \mathrm{HA(aq) + H_2O(l) \rightarrow H_3O^+(aq) + A^-(aq)} \)
Where \( \mathrm{HA} \) represents a strong acid such as HCl, HBr, HI, \( \mathrm{HNO_3} \), \( \mathrm{H_2SO_4} \), or \( \mathrm{HClO_4} \).
Key Idea:
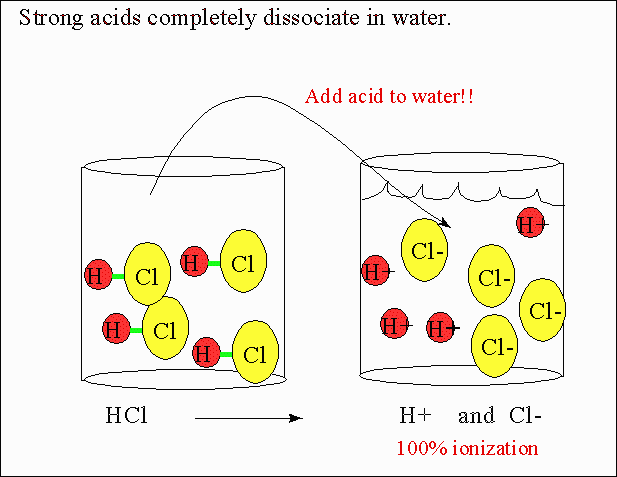
- Strong acids fully dissociate — there is no equilibrium between the acid and its ions.
- \( \mathrm{[H_3O^+]} = \) initial acid concentration (for monoprotic acids).
- For diprotic acids like \( \mathrm{H_2SO_4} \), the first ionization is complete, and the second contributes slightly more \( \mathrm{H_3O^+} \).
- Thus, pH can be calculated directly from the initial acid molarity.
Formula:
\( \mathrm{pH = -\log [H_3O^+]} \)
Example:
Determine the pH of a \( \mathrm{0.050\ M} \) \( \mathrm{HCl} \) solution.
▶️ Answer / Explanation
Step 1: Write the ionization equation:
\( \mathrm{HCl(aq) + H_2O(l) \rightarrow H_3O^+(aq) + Cl^-(aq)} \)
Step 2: Because HCl completely ionizes, \( \mathrm{[H_3O^+] = 0.050\ M} \).
Step 3: Calculate pH:
\( \mathrm{pH = -\log(0.050) = 1.30} \)
Final Answer: \( \mathrm{pH = 1.30} \)
Dissociation of Strong Bases
A strong base completely dissociates in aqueous solution to produce hydroxide ions (\( \mathrm{OH^-} \)). This means the \( \mathrm{[OH^-]} \) in the solution equals the initial base concentration for group 1 hydroxides (e.g., NaOH, KOH) and twice the initial concentration for group 2 hydroxides (e.g., Ca(OH)\(_2\), Ba(OH)\(_2\)) because they release two hydroxide ions per formula unit.
Dissociation Reactions:
- Group I hydroxide: \( \mathrm{NaOH(s) \rightarrow Na^+(aq) + OH^-(aq)} \)
- Group II hydroxide: \( \mathrm{Ba(OH)_2(s) \rightarrow Ba^{2+}(aq) + 2OH^-(aq)} \)
Key Relationships:
- For group I hydroxide: \( \mathrm{[OH^-] = [Base]_{initial}} \)
- For group II hydroxide: \( \mathrm{[OH^-] = 2 \times [Base]_{initial}} \)
- \( \mathrm{pOH = -\log [OH^-]} \)
- \( \mathrm{pH = 14.00 – pOH} \) at 25 °C
Example:
Calculate the pH of a \( \mathrm{0.010\ M} \) \( \mathrm{Ba(OH)_2} \) solution at 25 °C.
▶️ Answer / Explanation
Step 1: Write the dissociation equation:
\( \mathrm{Ba(OH)_2(s) \rightarrow Ba^{2+}(aq) + 2OH^-(aq)} \)
Step 2: Determine \( \mathrm{[OH^-]} \): \( \mathrm{[OH^-] = 2 \times 0.010 = 0.020\ M} \).
Step 3: Calculate pOH: \( \mathrm{pOH = -\log(0.020) = 1.70} \).
Step 4: Find pH: \( \mathrm{pH = 14.00 – 1.70 = 12.30} \).
Final Answer: \( \mathrm{pH = 12.30} \)
