AP Chemistry 8.3 Weak Acid and Base Equilibria Study Notes - New Syllabus Effective fall 2024
AP Chemistry 8.3 Weak Acid and Base Equilibria Study Notes- New syllabus
AP Chemistry 8.3 Weak Acid and Base Equilibria Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship among pH, pOH, and concentrations of all species in a solution of a monoprotic weak acid or weak base.
Key Concepts:
- Weak Acids and Partial Ionization
- Acid Dissociation Constant \( \mathrm{K_a} \) and \( \mathrm{pK_a} \)
- Weak Bases and Limited Ionization
- Base Dissociation Constant \( \mathrm{K_b} \) and \( \mathrm{pK_b} \)
- Percent Ionization of Weak Acids and Bases
- Relationship Between \( \mathrm{K_a} \), \( \mathrm{K_b} \), and \( \mathrm{K_w} \)
Weak Acids and Partial Ionization
Weak acids react with water to produce hydronium ions (\( \mathrm{H_3O^+} \)), but only a small fraction of the acid molecules actually ionize. Most of the acid remains in its molecular (un-ionized) form, leading to a low concentration of hydronium ions compared to the initial acid concentration.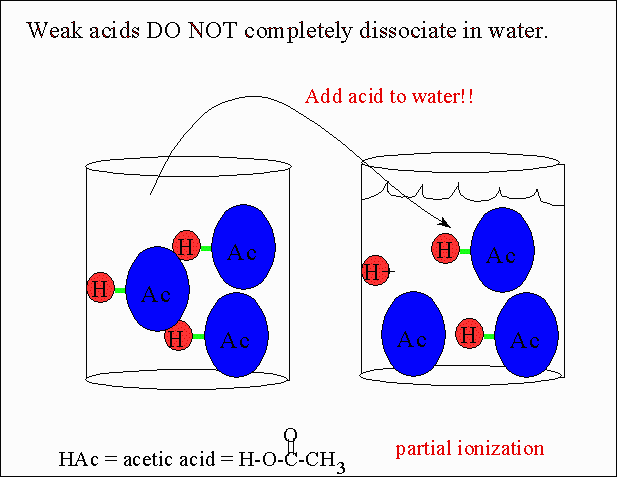
General Ionization Equation:
\( \mathrm{HA(aq) + H_2O(l) \rightleftharpoons H_3O^+(aq) + A^-(aq)} \)
- \( \mathrm{HA} \): weak acid
- \( \mathrm{A^-} \): conjugate base
- ⇌ represents an equilibrium — the reaction does not go to completion.
Key Characteristics:
- Only a small percentage (often less than 5%) of acid molecules ionize.
- \( \mathrm{[H_3O^+]} \) is much less than the initial acid concentration.
- Weak acids have higher pH values (less acidic) than strong acids of the same molarity.
Example:
Why is the pH of a 0.10 M acetic acid solution (CH₃COOH) higher than that of a 0.10 M HCl solution?
▶️ Answer / Explanation
Step 1: HCl is a strong acid — completely ionizes to give \( \mathrm{[H_3O^+] = 0.10\ M} \).
Step 2: CH₃COOH is a weak acid — only partially ionizes: \( \mathrm{CH_3COOH + H_2O \rightleftharpoons CH_3COO^- + H_3O^+} \)
Step 3: \( \mathrm{[H_3O^+]} \) from CH₃COOH is much smaller (≈ 1 × 10⁻³ M).
Final Answer: Because CH₃COOH ionizes less, its pH is higher (≈ 3) than HCl (pH ≈ 1).
Acid Dissociation Constant \( \mathrm{K_a} \) and \( \mathrm{pK_a} \)
A weak acid reaches equilibrium between the un-ionized acid (\( \mathrm{HA} \)) and its conjugate base (\( \mathrm{A^-} \)). The acid dissociation constant \( \mathrm{K_a} \) quantifies the extent of this ionization, while \( \mathrm{pK_a} \) provides a logarithmic measure of acid strength.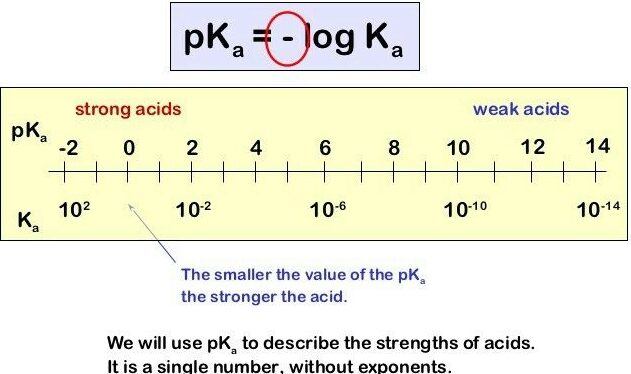
Equilibrium Expression:
\( \mathrm{K_a = \dfrac{[H_3O^+][A^-]}{[HA]}} \)
Relationship: \( \mathrm{pK_a = -\log K_a} \)
Interpretation:
- Smaller \( \mathrm{pK_a} \) → stronger acid (greater ionization).
- Larger \( \mathrm{pK_a} \) → weaker acid (less ionization).
Example:
The equilibrium constant for acetic acid is \( \mathrm{K_a = 1.8 \times 10^{-5}} \). Calculate its \( \mathrm{pK_a} \).
▶️ Answer / Explanation
Step 1: Use \( \mathrm{pK_a = -\log K_a} \).
Step 2: \( \mathrm{pK_a = -\log(1.8 \times 10^{-5}) = 4.74} \).
Final Answer: \( \mathrm{pK_a = 4.74} \) → moderately weak acid.
Weak Bases and Limited Ionization
Weak bases react with water to produce hydroxide ions (\( \mathrm{OH^-} \)), but only a small fraction of base molecules ionize. Most remain un-ionized, so the \( \mathrm{[OH^-]} \) is much lower than the base concentration.
General Reaction: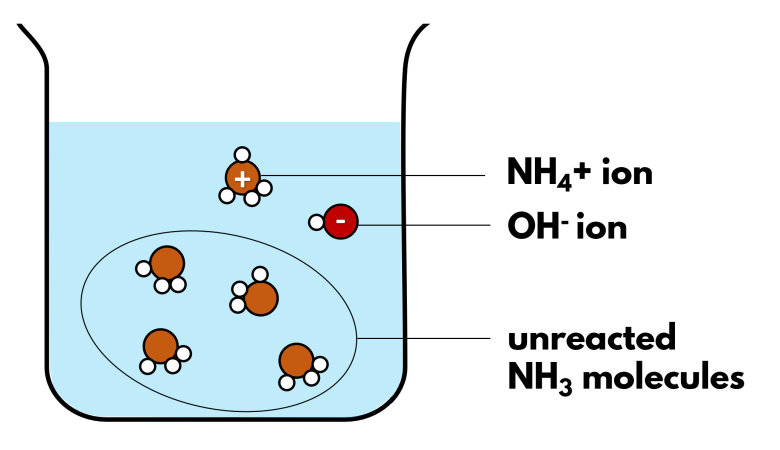
\( \mathrm{B(aq) + H_2O(l) \rightleftharpoons BH^+(aq) + OH^-(aq)} \)
- \( \mathrm{B} \): weak base (e.g., NH₃, CH₃NH₂)
- \( \mathrm{BH^+} \): conjugate acid
Key Points:
- Partial ionization → equilibrium established between base and its ions.
- \( \mathrm{[OH^-]} \ll [B]_{initial} \).
- Weak bases have lower pH values than strong bases of the same concentration.
Example:
Why is the pH of a 0.10 M ammonia solution (NH₃) lower than that of a 0.10 M NaOH solution?
▶️ Answer / Explanation
NaOH fully dissociates: \( \mathrm{[OH^-] = 0.10\ M} \).
NH₃ partially ionizes: \( \mathrm{NH_3 + H_2O \rightleftharpoons NH_4^+ + OH^-} \), giving \( \mathrm{[OH^-]} \approx 1 \times 10^{-3}\ M \).
Therefore, pH of NH₃ ≈ 11, much lower than NaOH (pH ≈ 13).
Base Dissociation Constant \( \mathrm{K_b} \) and \( \mathrm{pK_b} \)
Weak bases establish equilibrium between the base and its conjugate acid. The base ionization constant \( \mathrm{K_b} \) quantifies the extent of ionization; its logarithmic form \( \mathrm{pK_b} \) expresses base strength inversely.
Equilibrium Expression: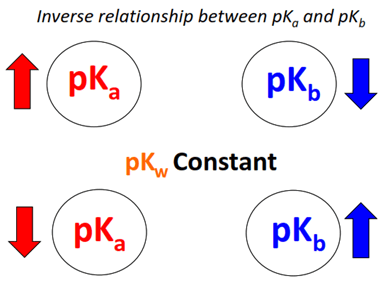
\( \mathrm{K_b = \dfrac{[OH^-][BH^+]}{[B]}} \)
Relationship: \( \mathrm{pK_b = -\log K_b} \)
Interpretation:
- Smaller \( \mathrm{pK_b} \) → stronger base (greater ionization).
- Larger \( \mathrm{pK_b} \) → weaker base (less ionization).
Example:
The base dissociation constant for ammonia is \( \mathrm{K_b = 1.8 \times 10^{-5}} \). Find \( \mathrm{pK_b} \).
▶️ Answer / Explanation
Step 1: \( \mathrm{pK_b = -\log(1.8 \times 10^{-5}) = 4.74} \)
Step 2: A smaller \( \mathrm{pK_b} \) means stronger base; NH₃ is weak but moderately basic.
Final Answer: \( \mathrm{pK_b = 4.74} \)
Percent Ionization of Weak Acids and Bases
Percent ionization indicates the fraction of acid or base molecules that ionize in solution. It depends on both the equilibrium constant (\( \mathrm{K_a} \) or \( \mathrm{K_b} \)) and the initial concentration of the species.
Formula:
\( \mathrm{\%\,Ionization = \dfrac{[Ionized]}{[Initial]} \times 100} \)
For acids: \( \mathrm{\%\,Ionization = \dfrac{[H_3O^+]}{[HA]_{initial}} \times 100} \)
For bases: \( \mathrm{\%\,Ionization = \dfrac{[OH^-]}{[B]_{initial}} \times 100} \)
Trends:
- Lower initial concentration → higher percent ionization (Le Châtelier’s principle).
- Stronger acid or base (larger \( \mathrm{K_a} \) or \( \mathrm{K_b} \)) → higher percent ionization.
Example:
A 0.10 M solution of acetic acid has \( \mathrm{[H_3O^+] = 1.3 \times 10^{-3}\ M} \). Calculate its percent ionization.
▶️ Answer / Explanation
\( \mathrm{\%\,Ionization = \dfrac{1.3\times10^{-3}}{0.10} \times 100 = 1.3\%} \)
Final Answer: Only 1.3% of CH₃COOH molecules ionize — confirming it is a weak acid.
Relationship Between \( \mathrm{K_a} \), \( \mathrm{K_b} \), and \( \mathrm{K_w} \)
For any conjugate acid–base pair, the strength of the acid and base are inversely related. Their ionization constants are connected by the ionic product of water (\( \mathrm{K_w} \)).
Mathematical Relationships: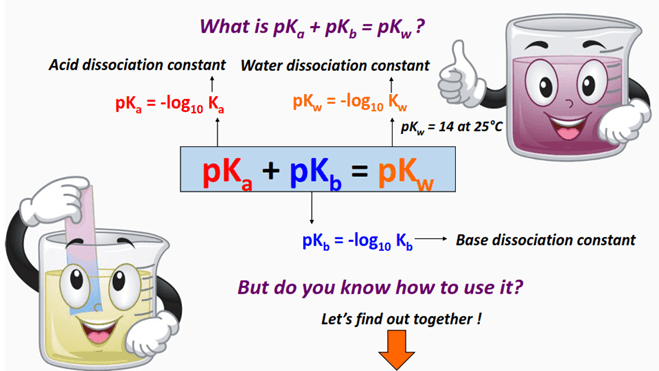
\( \mathrm{K_w = K_a \times K_b} \)
\( \mathrm{pK_w = pK_a + pK_b} \)
At 25 °C: \( \mathrm{K_w = 1.0 \times 10^{-14}} \) and \( \mathrm{pK_w = 14.00} \).
Interpretation:
- Strong acid → very weak conjugate base (\( \mathrm{K_a} \) large, \( \mathrm{K_b} \) small).
- Weak acid → relatively stronger conjugate base.
- Product \( \mathrm{K_a \times K_b} \) always equals \( \mathrm{K_w} \).
Example:
For acetic acid, \( \mathrm{K_a = 1.8 \times 10^{-5}} \). Find \( \mathrm{K_b} \) for its conjugate base, acetate ion.
▶️ Answer / Explanation
Step 1: \( \mathrm{K_b = \dfrac{K_w}{K_a}} = \dfrac{1.0\times10^{-14}}{1.8\times10^{-5}} = 5.6\times10^{-10}} \).
Step 2: \( \mathrm{pK_b = -\log(5.6\times10^{-10}) = 9.25} \).
Final Answer: Acetate ion is a weak base with \( \mathrm{K_b = 5.6\times10^{-10}} \).
