Acid Dissociation Constant
- (only) For weak acids:
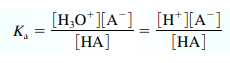 (note [H+] is the same as [H3O+])
(note [H+] is the same as [H3O+])- Strong acids don’t have Ka
Weak Acids
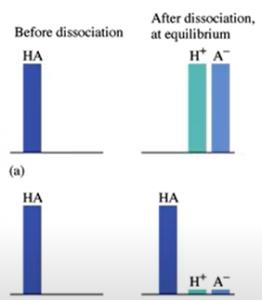
- Any acid that is not one of the 6
- They do not dissociate completely
- Equilibrium far to the left
- [H+ ] <<< [HA]
- A- is a stronger base than water
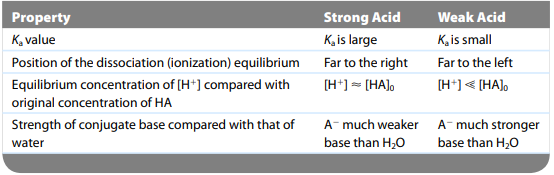
Strong vs Weak Acids
- If acid is very weak → conj. base is very strong
- If acid is very strong → conj. base is very weak
- Amount of H3O+ indicates how strong an acid is
Process to Calculate pH of (weak acid) Solutions
- Don’t memorize → instead know the chemistry bcuz there is no ONE way to do these problems
1. List major species in solution
2. Choose species that can produce H+ and write reactions
- Will sometimes have to first write out net ionic equation → will reveal another equation that produces [H+] or [OH-]
3. Based on K values, decide on dominant equilibrium
4. Write equilibrium expression for dominant equilibrium
5. List initial concentrations in dominant equilibrium
6. Define change at equilibrium (as X)
7. Write equilibrium concentrations in terms of x
8. Plug in equilibrium concentrations into eq expression
9. Find the intersection between the eq expression and Ka value graphically
10. Calculate [H+] and pH
Practice Notes
- If are in a MCQ situation
Weak Acids
- Ka (given) will be small → a weaker acid will produce fewer H3O+ ions and thus a higher (less acidic) pH
- Always write down the major species! → then use ICE Tables
- Now x is the [OH-] → easier to find the pOH first
Percent Dissociation
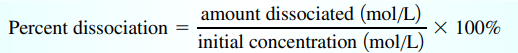
- 100% for strong acids
- Numerator = x; denominator = []o /
- For a weak acid, percent dissociation increases as an acid becomes more dilute
- As [HA]₀ decreases, [H+] decreases but % dissociation increases
- Note: Percent dissociation → acids; Percent ionization → bases
- Are set up the same way
Summary
Polyprotic Acids
- A polyprotic acid always dissociates in a stepwise manner, one proton at a time
- Ex:
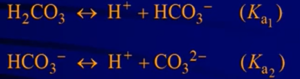

- Ex:
- In calculations, can normally ignore the 2nd (& sometimes third) dissociations bcuz the first is much larger (ie. donates most of the H+)
- Focus attention & write an ICE table for only the first equation Ka value
