AP Chemistry 8.4 Acid-Base Reactions and Buffers Study Notes - New Syllabus Effective fall 2024
AP Chemistry 8.4 Acid-Base Reactions and Buffers Study Notes- New syllabus
AP Chemistry 8.4 Acid-Base Reactions and Buffers Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship among the concentrations of major species in a mixture of weak and strong acids and bases.
Key Concepts:
- Reaction Between a Strong Acid and a Strong Base
- Reaction Between a Weak Acid and a Strong Base
- Reaction Between a Weak Base and a Strong Acid
- Reaction Between a Weak Acid and a Weak Base
Reaction Between a Strong Acid and a Strong Base
When a strong acid and a strong base are mixed, they neutralize each other completely. Both substances dissociate fully in aqueous solution, and their ions combine to form water.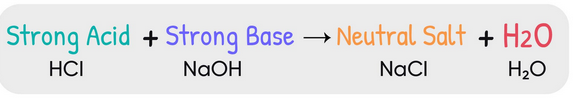
Reaction Equation:
\( \mathrm{H^+(aq) + OH^-(aq) \rightarrow H_2O(l)} \)
This reaction is quantitative — it goes to completion, leaving only water and any excess reagent in solution.
Determining pH:
- If equal moles of acid and base react → solution is neutral (pH = 7 at 25 °C).
- If one reagent is in excess → pH is determined by the concentration of excess H⁺ or OH⁻ after reaction.
Example:
Calculate the pH when 50.0 mL of 0.10 M HCl reacts with 25.0 mL of 0.10 M NaOH.
▶️ Answer / Explanation
Step 1: Find moles of each reagent.
\( \mathrm{n_{HCl} = 0.050 \times 0.10 = 0.0050\ mol} \)
\( \mathrm{n_{NaOH} = 0.025 \times 0.10 = 0.0025\ mol} \)
Step 2: Determine excess species.
HCl is in excess → \( \mathrm{0.0050 – 0.0025 = 0.0025\ mol\ H^+} \) remain.
Step 3: Calculate concentration of \( \mathrm{H^+} \).
Total volume = 75.0 mL = 0.075 L.
\( \mathrm{[H^+] = \dfrac{0.0025}{0.075} = 0.0333\ M} \)
Step 4: Find pH.
\( \mathrm{pH = -\log(0.0333) = 1.48} \)
Final Answer: pH = 1.48 (acidic due to excess HCl).
Reaction Between a Weak Acid and a Strong Base
When a weak acid reacts with a strong base, neutralization occurs between the hydronium-producing weak acid and the hydroxide ions of the strong base. The reaction produces the conjugate base of the weak acid and water.
Reaction Equation: 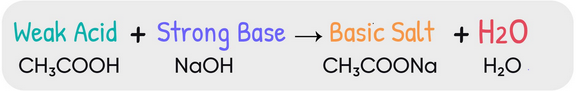
\( \mathrm{HA(aq) + OH^-(aq) \rightarrow A^-(aq) + H_2O(l)} \)
pH Determination:
- If the weak acid is in excess → solution becomes a buffer (use Henderson–Hasselbalch equation).
- If the strong base is in excess → pH is determined by remaining \( \mathrm{[OH^-]} \).
- If equimolar → solution contains only the conjugate base → slightly basic (due to hydrolysis).
Hydrolysis of the Conjugate Base:
\( \mathrm{A^-(aq) + H_2O(l) \rightleftharpoons HA(aq) + OH^-(aq)} \)
Example:
Determine whether the final solution is acidic, basic, or neutral when equal moles of 0.10 M CH₃COOH and 0.10 M NaOH are mixed.
▶️ Answer / Explanation
Step 1: Reaction: \( \mathrm{CH_3COOH + OH^- \rightarrow CH_3COO^- + H_2O} \)
Step 2: Equal moles → all acid neutralized → only acetate ions remain.
Step 3: Acetate ions undergo hydrolysis: \( \mathrm{CH_3COO^- + H_2O \rightleftharpoons CH_3COOH + OH^-} \)
Step 4: \( \mathrm{[OH^-]} \) increases slightly → pH > 7.
Final Answer: Solution is basic (slightly, pH ≈ 8.5).
Reaction Between a Weak Base and a Strong Acid
When a weak base reacts with a strong acid, neutralization occurs through proton transfer from the acid to the base, forming the conjugate acid of the base and water.
Reaction Equation: 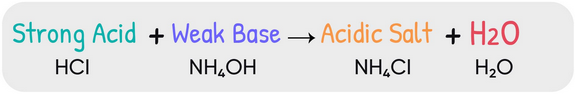
\( \mathrm{B(aq) + H_3O^+(aq) \rightarrow BH^+(aq) + H_2O(l)} \)
pH Determination:
- If the weak base is in excess → buffer solution forms (use Henderson–Hasselbalch equation).
- If the strong acid is in excess → pH from remaining \( \mathrm{[H_3O^+]} \).
- If equimolar → conjugate acid \( \mathrm{BH^+} \) hydrolyzes slightly → pH < 7.
Hydrolysis of Conjugate Acid:
\( \mathrm{BH^+(aq) + H_2O(l) \rightleftharpoons B(aq) + H_3O^+(aq)} \)
Example:
What is the pH when 0.10 M NH₃ reacts with 0.10 M HCl in equal moles?
▶️ Answer / Explanation
Step 1: \( \mathrm{NH_3 + H_3O^+ \rightarrow NH_4^+ + H_2O} \)
Step 2: Equal moles → only \( \mathrm{NH_4^+} \) remains.
Step 3: \( \mathrm{NH_4^+} \) hydrolyzes: \( \mathrm{NH_4^+ + H_2O \rightleftharpoons NH_3 + H_3O^+} \)
Step 4: \( \mathrm{H_3O^+} \) formed → pH < 7.
Final Answer: Solution is acidic (pH ≈ 5.5).
Reaction Between a Weak Acid and a Weak Base
When a weak acid and a weak base are mixed, they react incompletely and reach an equilibrium state. The extent of reaction depends on the relative strengths of the acid and base involved.
Reaction Equation: 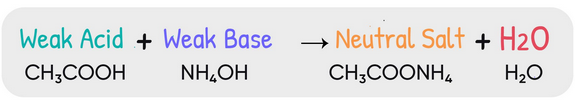
\( \mathrm{HA(aq) + B(aq) \rightleftharpoons A^-(aq) + HB^+(aq)} \)
pH Determination:
- If \( \mathrm{K_a > K_b} \) → solution is acidic (pH < 7).
- If \( \mathrm{K_b > K_a} \) → solution is basic (pH > 7).
- If \( \mathrm{K_a \approx K_b} \) → solution is nearly neutral.
Example:
Predict the pH of a solution formed by mixing acetic acid (\( \mathrm{K_a = 1.8 \times 10^{-5}} \)) and ammonia (\( \mathrm{K_b = 1.8 \times 10^{-5}} \)).
▶️ Answer / Explanation
Step 1: \( \mathrm{CH_3COOH + NH_3 \rightleftharpoons CH_3COO^- + NH_4^+} \)
Step 2: \( \mathrm{K_a = K_b} \) → strengths are equal → equilibrium near neutral point.
Step 3: Solution pH ≈ 7 (slightly shifted depending on concentration).
Final Answer: Solution is approximately neutral.
