AP Chemistry 8.6 Molecular Structure of Acids and Bases Study Notes - New Syllabus Effective fall 2024
AP Chemistry 8.6 Molecular Structure of Acids and Bases Study Notes- New syllabus
AP Chemistry 8.6 Molecular Structure of Acids and Bases Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship between the strength of an acid or base and the structure of the molecule or ion.
Key Concepts:
- Relationship Between Acid/Base Strength and Molecular Structure
Relationship Between Acid/Base Strength and Molecular Structure
The strength of an acid or base depends on how easily it can donate or accept a proton, which in turn depends on the stability of its conjugate species. Structural features such as bond polarity, bond strength, resonance, and electronegativity influence how readily a proton is transferred in an acid–base reaction.
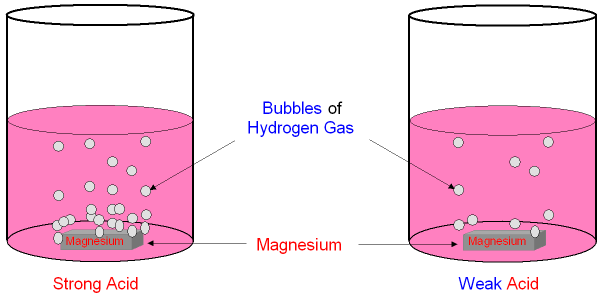
1. Strength of Acids and Conjugate Bases
- Strong acids (e.g., HCl, HBr, HI, \( \mathrm{HClO_4} \), \( \mathrm{H_2SO_4} \), \( \mathrm{HNO_3} \)) dissociate completely in water and have very weak conjugate bases.
- The conjugate bases of strong acids are highly stabilized by:
- High electronegativity of the attached atom,
- Inductive effects from nearby electronegative groups,
- Resonance delocalization of negative charge, or
- Large atomic size distributing charge over greater volume (as in HI).
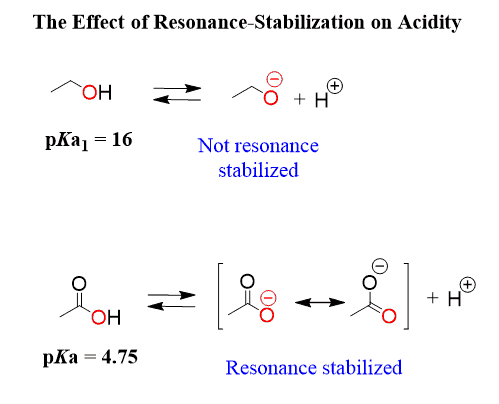
2. Weak Acids
- Carboxylic acids (e.g., \( \mathrm{CH_3COOH} \)) are typical weak acids.
- They partially dissociate because the conjugate base (carboxylate ion) is stabilized by resonance but not completely delocalized or electronegatively strong enough to make the acid fully dissociate.
- Acid strength increases with more electronegative atoms near the –COOH group (e.g., \( \mathrm{CH_2ClCOOH} \) > \( \mathrm{CH_3COOH} \)).
3. Strength of Bases and Conjugate Acids
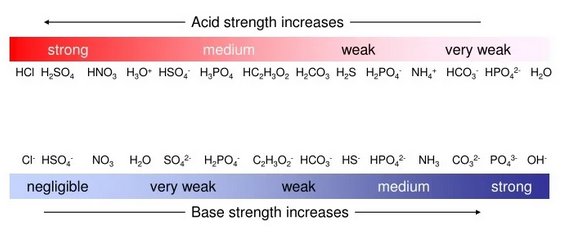
- Strong bases (e.g., \( \mathrm{NaOH} \), \( \mathrm{KOH} \), \( \mathrm{Ca(OH)_2} \)) dissociate completely to produce \( \mathrm{OH^-} \) ions.
- The conjugate acids of these bases (e.g., \( \mathrm{H_2O} \)) are very weak acids.
- Weak bases such as ammonia (\( \mathrm{NH_3} \)) or amines partially accept protons because their conjugate acids (\( \mathrm{NH_4^+} \)) are only moderately stable.
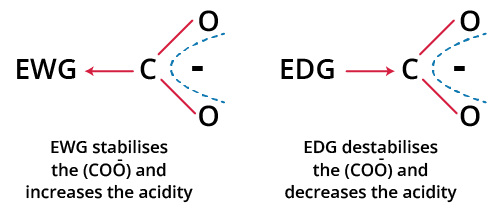
4. Role of Electronegative and Resonance Effects
- Electronegativity: Electronegative atoms near the acidic hydrogen pull electron density away, stabilizing the conjugate base and increasing acidity.
- Resonance: Delocalization of negative charge across multiple atoms stabilizes the conjugate base and strengthens the acid.
- Inductive effects: Electron-withdrawing substituents (–F, –Cl, –NO₂) stabilize conjugate bases through sigma bonds.
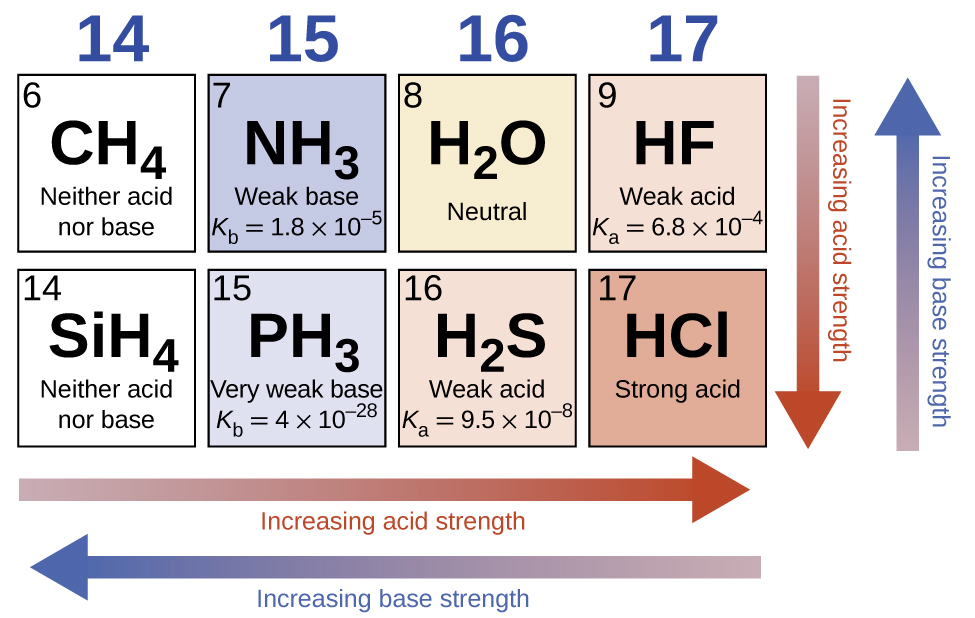
5. Periodic Trends in Acid Strength
- Across a period: Acid strength increases with increasing electronegativity (HF < HClO < HClO₂ < HClO₃ < HClO₄).
- Down a group: Acid strength increases with increasing atomic size (HF < HCl < HBr < HI).
Example:
Which acid is stronger: \( \mathrm{CH_3COOH} \) or \( \mathrm{CH_2ClCOOH} \)? Explain based on structure.
▶️ Answer / Explanation
Step 1: Both are carboxylic acids with the general form \( \mathrm{R–COOH} \).
Step 2: In \( \mathrm{CH_2ClCOOH} \), the chlorine atom is highly electronegative and exerts an inductive effect, pulling electron density away from the –COOH group.
Step 3: This stabilizes the conjugate base (\( \mathrm{CH_2ClCOO^-} \)) by delocalizing its negative charge.
Final Answer: \( \mathrm{CH_2ClCOOH} \) is a stronger acid than \( \mathrm{CH_3COOH} \) due to the electron-withdrawing inductive effect of chlorine.
Example :
Which acid is stronger — \( \mathrm{CH_3COOH} \) (acetic acid) or \( \mathrm{CH_2ClCOOH} \) (chloroacetic acid)? Explain based on molecular structure.
▶️ Answer / Explanation
Step 1: Both are carboxylic acids that ionize as:
\( \mathrm{RCOOH \rightleftharpoons RCOO^- + H^+} \)
Step 2: In chloroacetic acid (\( \mathrm{CH_2ClCOOH} \)), the chlorine atom is highly electronegative and withdraws electron density from the –COOH group via the inductive effect.
Step 3: This stabilizes the conjugate base (\( \mathrm{CH_2ClCOO^-} \)) by delocalizing negative charge, making the acid stronger.
Step 4: In acetic acid (\( \mathrm{CH_3COOH} \)), the methyl group donates electrons, slightly destabilizing the conjugate base.
Final Answer: \( \mathrm{CH_2ClCOOH} \) is the stronger acid because the electronegative chlorine atom stabilizes the conjugate base through inductive effects.
Example :
Which of the following is the stronger acid — \( \mathrm{HF} \) or \( \mathrm{HI} \)? Explain using molecular structure and periodic trends.
▶️ Answer / Explanation
Step 1: Both are hydrogen halides that ionize as:
\( \mathrm{HX \rightleftharpoons H^+ + X^-} \)
Step 2: Down Group 17, the bond strength between H and X decreases (H–F is very strong, H–I is weak).
Step 3: The weaker H–I bond breaks more easily, and the larger iodide ion (\( \mathrm{I^-} \)) disperses its negative charge more effectively than the smaller fluoride ion (\( \mathrm{F^-} \)).
Final Answer: \( \mathrm{HI} \) is a much stronger acid than \( \mathrm{HF} \) because it has a weaker H–I bond and a more stable conjugate base (\( \mathrm{I^-} \)).