AP Chemistry 8.7 pH and pKa Study Notes - New Syllabus Effective fall 2024
AP Chemistry 8.7 pH and pKa Study Notes- New syllabus
AP Chemistry 8.7 pH and pKa Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship between the predominant form of a weak acid or base in solution at a given pH and the pKa of the conjugate acid or the pKb of the conjugate base
Key Concepts:
- Relationship Between pH, pKa, and Protonation State
- Acid–Base Indicators and Protonation
- Selecting an Appropriate Indicator for a Titration
Relationship Between pH, pKa, and Protonation State
The protonation state of an acid or base—whether it exists mostly in its acid form (HA) or base form (A⁻)—can be predicted by comparing the pH of the solution to the pKa of the acid.
Key Relationship: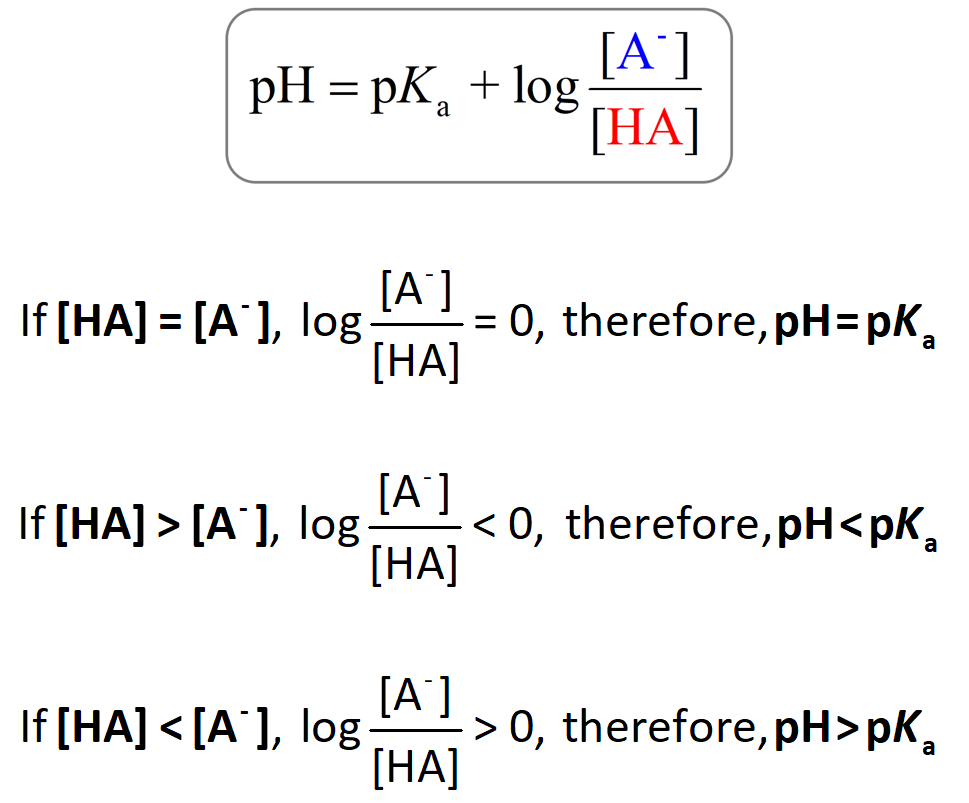
- If \( \mathrm{pH < pK_a} \): the solution is more acidic → the acidic form (HA) predominates.
- If \( \mathrm{pH > pK_a} \): the solution is more basic → the basic (deprotonated) form (A⁻) predominates.
- If \( \mathrm{pH = pK_a} \): the concentrations of HA and A⁻ are equal → 50% protonated, 50% deprotonated.
Henderson–Hasselbalch Equation:
\( \mathrm{pH = pK_a + \log\!\left(\dfrac{[A^-]}{[HA]}\right)} \)
Interpretation:
- When \( \mathrm{[A^-] > [HA]} \), the solution is more basic (pH > pKa).
- When \( \mathrm{[HA] > [A^-]} \), the solution is more acidic (pH < pKa).
Example:
For acetic acid (\( \mathrm{pK_a = 4.76} \)), what is the predominant species at pH = 3.76 and pH = 6.76?
▶️ Answer / Explanation
At pH = 3.76: \( \mathrm{pH < pK_a} \) → mostly \( \mathrm{HA} \) (protonated acetic acid).
At pH = 6.76: \( \mathrm{pH > pK_a} \) → mostly \( \mathrm{A^-} \) (acetate ion).
At pH = 4.76: \( \mathrm{pH = pK_a} \) → 50% \( \mathrm{HA} \), 50% \( \mathrm{A^-} \).
Acid–Base Indicators and Protonation
Acid–base indicators are weak acids or bases that exhibit different observable properties—such as color—in their protonated and deprotonated forms. The visible color change occurs when the relative concentrations of the two forms become comparable.
General Reaction of an Indicator: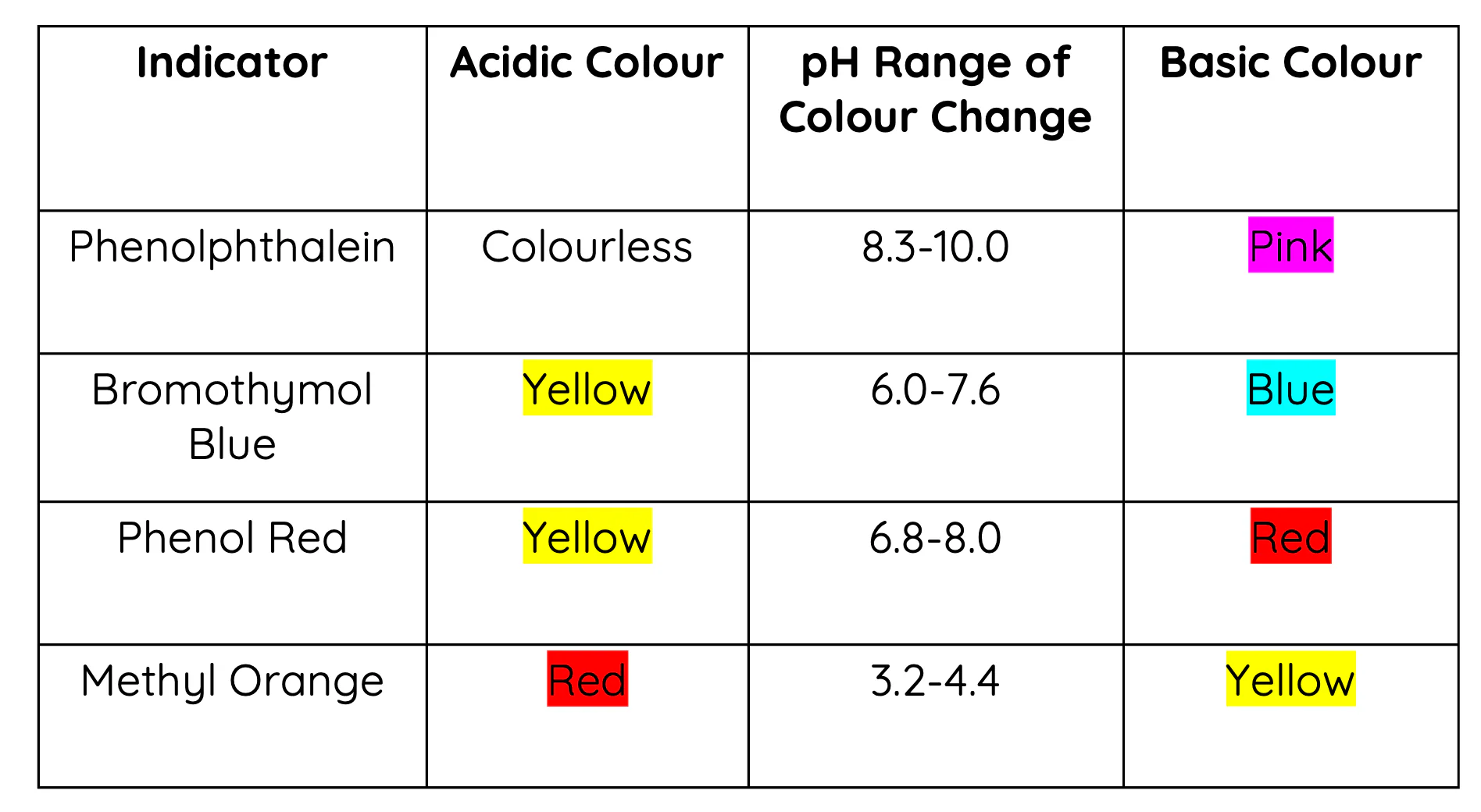
\( \mathrm{HIn(aq) \rightleftharpoons H^+(aq) + In^-(aq)} \)
- \( \mathrm{HIn} \): protonated form (one color)
- \( \mathrm{In^-} \): deprotonated form (different color)
Color Change Range:
- Occurs approximately between \( \mathrm{pH = pK_a \pm 1} \).
- At \( \mathrm{pH < pK_a} \) → color of \( \mathrm{HIn} \) (acidic form).
- At \( \mathrm{pH > pK_a} \) → color of \( \mathrm{In^-} \) (basic form).
Example:
Describe the color changes of phenolphthalein (pKa ≈ 9.3) as pH changes from 7 to 11.
▶️ Answer / Explanation
Below pH 8.3: \( \mathrm{pH < pK_a} \) → protonated form \( \mathrm{HIn} \) → colorless.
Between pH 8.3–10.3: mixture of forms → pink transition range.
Above pH 10.3: \( \mathrm{pH > pK_a} \) → deprotonated form \( \mathrm{In^-} \) → bright pink.
Selecting an Appropriate Indicator for a Titration
For a titration experiment to yield accurate results, the acid–base indicator used must have a \( \mathrm{pK_a} \) value close to the pH at the equivalence point of the titration. This ensures that the indicator changes color precisely when neutralization is complete.
Selection Criteria:
Choose an indicator whose transition range (pKa ± 1) includes the pH at the equivalence point.
- Different titrations require different indicators:
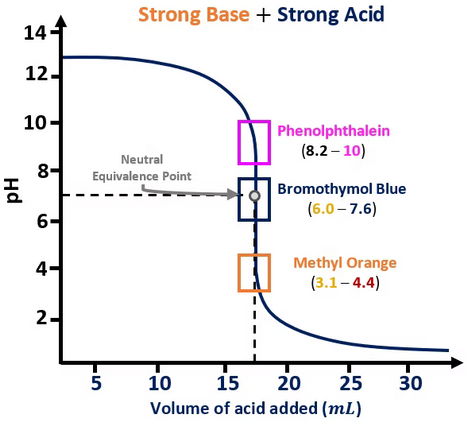
- Strong acid–strong base: pH ≈ 7 → indicators like bromothymol blue (pKa ≈ 7.1).
- Weak acid–strong base: pH > 7 → indicators like phenolphthalein (pKa ≈ 9.3).
- Weak base–strong acid: pH < 7 → indicators like methyl orange (pKa ≈ 3.7).
Example:
Which indicator should be used for the titration of 0.10 M acetic acid with 0.10 M NaOH?
▶️ Answer / Explanation
Step 1: For a weak acid–strong base titration, pH at equivalence ≈ 8.5.
Step 2: Choose indicator with transition range near pH 8–10.
Final Answer: Phenolphthalein is ideal (color change from colorless → pink between pH 8.3–10.3).
