AP Chemistry 8.8 Properties of Buffers Study Notes - New Syllabus Effective fall 2024
AP Chemistry 8.8 Properties of Buffers Study Notes- New syllabus
AP Chemistry 8.8 Properties of Buffers Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationshipbetween the ability of abuffer to stabilize pH and thereactions that occur when anacid or a base is added to abuffered solution.
Key Concepts:
- Buffer Systems and pH Stabilization
Buffer Systems and pH Stabilization
A buffer solution is a mixture that contains a large concentration of both a weak acid and its conjugate base (or a weak base and its conjugate acid). It resists significant changes in pH when small amounts of acid or base are added, because the conjugate pair reacts with the added species to neutralize it.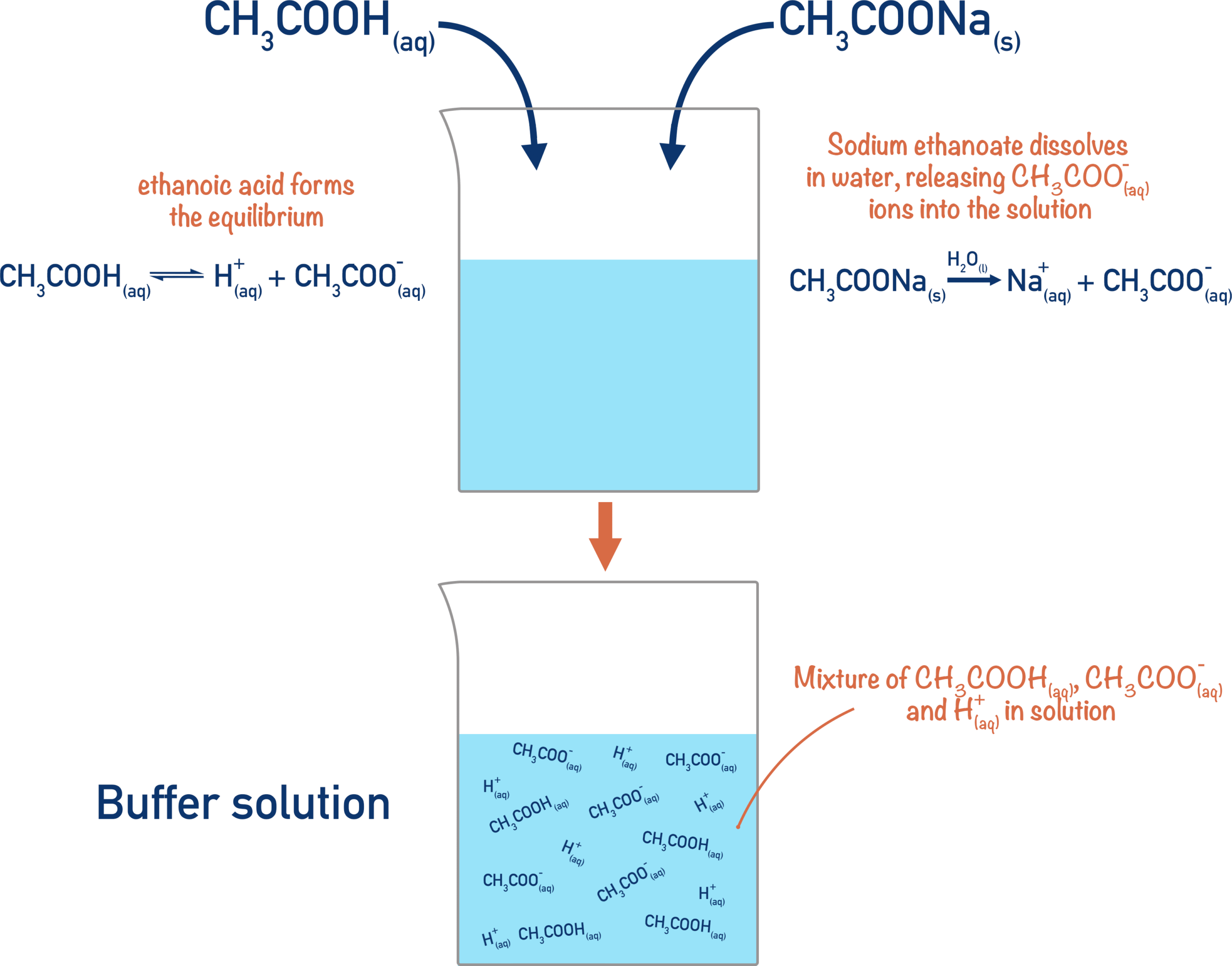
Key Components:
- Weak acid (HA) — neutralizes added base by donating protons.
- Conjugate base (A⁻) — neutralizes added acid by accepting protons.
Buffer Reactions:
- When acid is added: \( \mathrm{A^-(aq) + H_3O^+(aq) \rightarrow HA(aq) + H_2O(l)} \)
- When base is added: \( \mathrm{HA(aq) + OH^-(aq) \rightarrow A^-(aq) + H_2O(l)} \)
These reactions consume most of the added \( \mathrm{H_3O^+} \) or \( \mathrm{OH^-} \), preventing large changes in the hydronium or hydroxide ion concentration and therefore stabilizing pH.
Quantitative Relationship — Henderson–Hasselbalch Equation:
\( \mathrm{pH = pK_a + \log\!\left(\dfrac{[A^-]}{[HA]}\right)} \)
The ratio \( \mathrm{[A^-]/[HA]} \) determines the buffer’s pH. As long as both species are present in comparable amounts, the pH remains relatively constant.
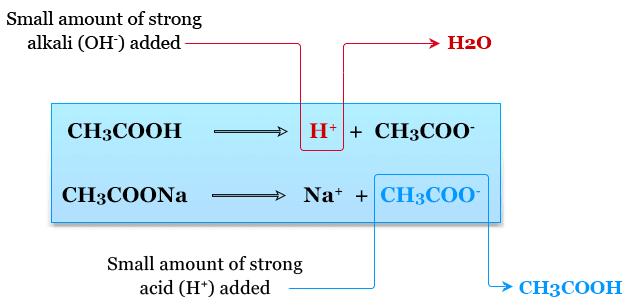
Key Idea:
- Buffers stabilize pH by converting strong acids and bases into weak ones.
- The effectiveness of a buffer (buffer capacity) depends on the absolute and relative concentrations of \( \mathrm{[A^-]} \) and \( \mathrm{[HA]} \).
- Maximum buffering occurs when \( \mathrm{pH = pK_a} \), because \( \mathrm{[A^-] = [HA]} \).
Example :
Explain how a buffer made of \( \mathrm{CH_3COOH} \) and \( \mathrm{CH_3COONa} \) resists a decrease in pH when a small amount of strong acid (HCl) is added.
▶️ Answer / Explanation
Step 1: The added \( \mathrm{H^+} \) ions react with acetate ions \( \mathrm{CH_3COO^-} \):
\( \mathrm{CH_3COO^-(aq) + H^+(aq) \rightarrow CH_3COOH(aq)} \)
Step 2: Most added \( \mathrm{H^+} \) is neutralized; the concentration of hydronium ions increases only slightly.
Final Answer: The pH decreases only marginally — the buffer resists acid addition by converting base form into acid form.
Example :
Describe what happens when NaOH is added to the same acetic acid/acetate buffer.
▶️ Answer / Explanation
Step 1: The hydroxide ions from NaOH react with acetic acid:
\( \mathrm{CH_3COOH(aq) + OH^-(aq) \rightarrow CH_3COO^-(aq) + H_2O(l)} \)
Step 2: The base is neutralized and replaced by additional acetate ions.
Final Answer: The pH rises only slightly — the buffer converts the strong base into the weak conjugate base.
Example :
Explain how the carbonic acid–bicarbonate buffer system maintains blood pH near 7.4.
▶️ Answer / Explanation
Step 1: The buffer pair is \( \mathrm{H_2CO_3} \) (acid) and \( \mathrm{HCO_3^-} \) (base).
Step 2: When acid is added:
\( \mathrm{HCO_3^- + H^+ \rightarrow H_2CO_3} \)
Step 3: When base is added:
\( \mathrm{H_2CO_3 + OH^- \rightarrow HCO_3^- + H_2O} \)
Step 4: The buffer minimizes changes in blood pH despite metabolic acid/base production.
Final Answer: The carbonic acid–bicarbonate system maintains blood pH ≈ 7.4 by neutralizing added acids or bases through reversible reactions.
