AP Chemistry 9.4 Thermodynamic and Kinetic Control Study Notes - New Syllabus Effective fall 2024
AP Chemistry 9.4 Thermodynamic and Kinetic Control Study Notes- New syllabus
AP Chemistry 9.4 Thermodynamic and Kinetic Control Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain, in terms of kinetics, why a thermodynamically favored reaction might not occur at a measurable rate.
Key Concepts:
- Thermodynamically Favored Processes and Reaction Rate
- Processes Under Kinetic Control
Thermodynamically Favored Processes and Reaction Rate
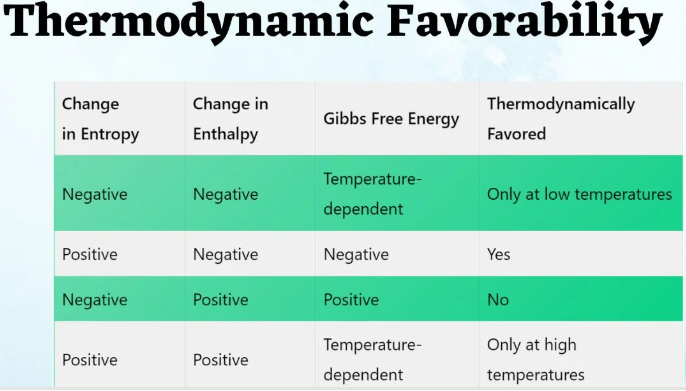
Some processes that are thermodynamically favored (i.e., have a negative Gibbs free energy change, \( \mathrm{\Delta G < 0} \)) do not occur to any measurable extent or occur extremely slowly. This distinction arises because thermodynamics determines spontaneity, while kinetics determines the rate at which the process actually happens.
- Thermodynamic favorability only tells whether a process can occur, not whether it will occur quickly.
- Even if \( \mathrm{\Delta G < 0} \), the process may proceed so slowly that it appears not to occur.
- The rate of reaction depends on the activation energy and reaction mechanism.
Key Relationship
\( \mathrm{\Delta G = \Delta H – T\Delta S} \)
- If \( \mathrm{\Delta G < 0} \) → the process is thermodynamically favored (spontaneous).
- However, spontaneity does not imply a fast rate — kinetics governs how quickly equilibrium is reached.
Key Concept Summary
- Thermodynamics → tells if a process is energetically favorable.
- Kinetics → tells how quickly the process occurs.
- Some spontaneous reactions are so slow that they seem nonspontaneous under normal conditions.
Example
The rusting of iron is a spontaneous process (\( \mathrm{\Delta G < 0} \)), but a clean iron nail takes weeks or months to rust. Explain why this happens.
▶️ Answer / Explanation
Step 1: The process is thermodynamically favored because it releases energy (\( \mathrm{\Delta G < 0} \)).
Step 2: However, forming the rust layer involves several redox reactions with high activation energy.
Step 3: These slow steps make the overall reaction occur at a very slow rate, even though it is spontaneous.
Final Answer: The rusting of iron is spontaneous but kinetically slow due to a large activation energy barrier.
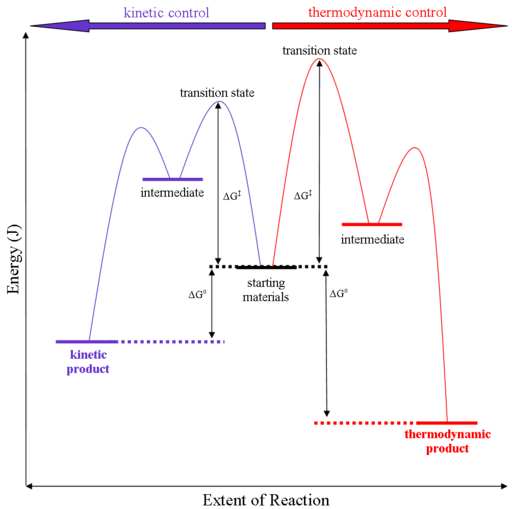
Processes Under Kinetic Control
Processes that are thermodynamically favored but occur at an immeasurably slow rate are said to be under kinetic control. A high activation energy (Eₐ) prevents the system from readily proceeding toward the thermodynamically stable state.
- Such reactions do not reach equilibrium quickly because the required energy barrier is too high.
- The chemical system may remain in a metastable or less stable state for a long time.
- Increasing temperature or using a catalyst can lower \( \mathrm{E_a} \) and make the reaction proceed faster.
Key Relationship
\( \mathrm{k = Ae^{-\frac{E_a}{RT}}} \)
- This is the Arrhenius equation, showing that a higher \( \mathrm{E_a} \) → smaller rate constant \( \mathrm{k} \) → slower reaction.
- A thermodynamically favored process can have a small \( \mathrm{k} \) if \( \mathrm{E_a} \) is large.
Key Concept Summary
- Kinetic control: Reaction is spontaneous but proceeds very slowly due to high activation energy.
- Thermodynamic control: Reaction direction and final equilibrium position are determined by \( \mathrm{\Delta G} \).
- “No reaction observed” ≠ “equilibrium achieved.” A process under kinetic control is simply stuck behind an energy barrier.
Example
Diamond is thermodynamically less stable than graphite at standard conditions, yet it does not spontaneously convert to graphite. Explain why diamond is stable at room temperature.
▶️ Answer / Explanation
Step 1: The reaction \( \mathrm{C_{diamond} \rightarrow C_{graphite}} \) has \( \mathrm{\Delta G < 0} \), so it is thermodynamically favored.
Step 2: However, breaking strong covalent bonds in diamond requires a very high activation energy.
Step 3: At room temperature, very few carbon atoms have enough energy to overcome this barrier.
Step 4: Thus, the reaction is under kinetic control — it is spontaneous but occurs at an immeasurably slow rate.
Final Answer: Diamond remains stable at room temperature because the conversion to graphite is thermodynamically favored but kinetically hindered due to an extremely high activation energy.
