AP Chemistry 9.10 Cell Potential Under Nonstandard Conditions Study Notes - New Syllabus Effective fall 2024
AP Chemistry 9.10 Cell Potential Under Nonstandard Conditions Study Notes- New syllabus
AP Chemistry 9.10 Cell Potential Under Nonstandard Conditions Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship between deviations from standard cell conditions and changes in the cell potential.
Key Concepts:
- Cell Potential Under Nonstandard Conditions
- Why Le Châtelier’s Principle Does Not Apply to Electrochemical Systems
- Relationship Between Cell Potential, \( \mathrm{Q} \), and Equilibrium
- Qualitative Understanding of the Nernst Equation and Concentration Effects
Cell Potential Under Nonstandard Conditions
In a real electrochemical system, the cell potential (\( \mathrm{E_{cell}} \)) depends on the concentrations (or partial pressures) of the reactants and products involved in the redox process. When the system is not under standard conditions (\( \mathrm{1\ M} \) solutions, \( \mathrm{1\ atm} \) gases, and \( \mathrm{298\ K} \)), the measured potential will differ from the standard cell potential (\( \mathrm{E^\circ_{cell}} \)).
The cell potential acts as the driving force that pushes the system toward equilibrium. The farther the system is from equilibrium, the larger the magnitude of the cell potential.
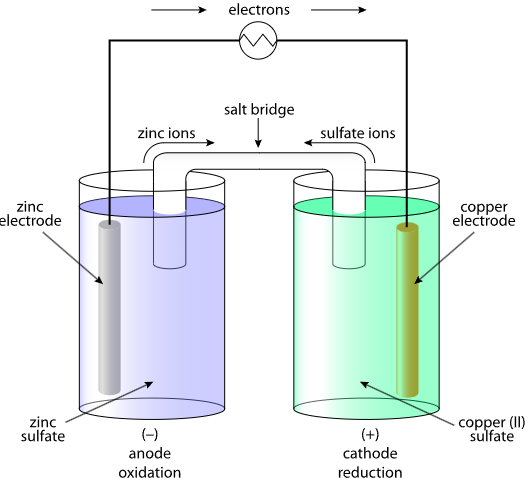
Key Relationship: Reaction Quotient \( \mathrm{Q} \)
The reaction quotient \( \mathrm{Q} \) describes the ratio of product to reactant concentrations for the overall redox reaction at a given instant:
\( \mathrm{Q = \dfrac{[Products]^{coeff}}{[Reactants]^{coeff}}} \)
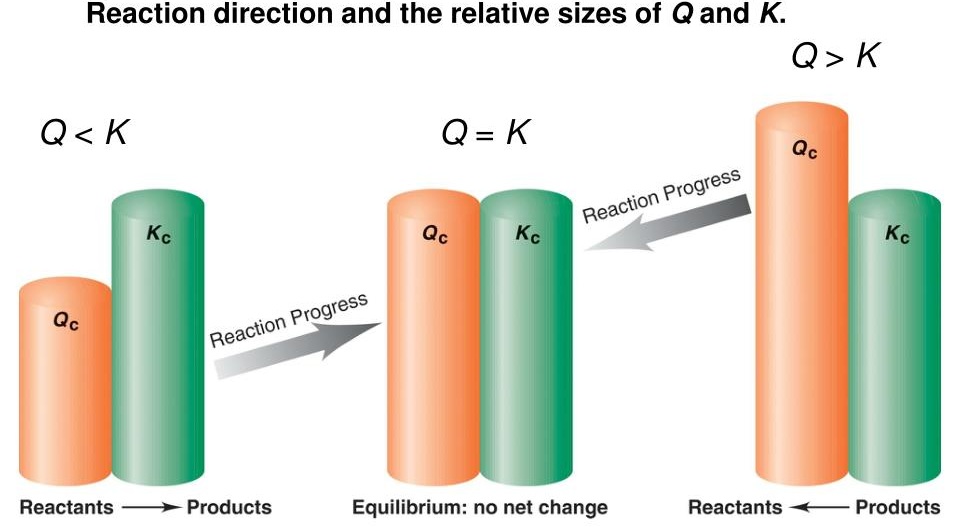
- When \( \mathrm{Q < K} \): reaction is spontaneous forward, \( \mathrm{E_{cell} > 0} \)
- When \( \mathrm{Q = K} \): system at equilibrium, \( \mathrm{E_{cell} = 0} \)
- When \( \mathrm{Q > K} \): reaction is nonspontaneous forward, \( \mathrm{E_{cell} < 0} \)
Conceptual Connection:
- At standard conditions (\( \mathrm{Q = 1} \)), the cell potential equals \( \mathrm{E^\circ_{cell}} \).
- When reactants are present in higher concentration (\( \mathrm{Q < 1} \)), the cell potential increases — system far from equilibrium.
- When products build up (\( \mathrm{Q > 1} \)), the cell potential decreases — system moves toward equilibrium.
Qualitative Relationship to Equilibrium
Cell potential is the measurable manifestation of how far a redox reaction is from equilibrium.
| System Condition | Value of \( \mathrm{Q} \) | Effect on \( \mathrm{E_{cell}} \) | Reason |
|---|---|---|---|
| Far from equilibrium (many reactants) | \( \mathrm{Q < 1} \) | High \( \mathrm{E_{cell}} \) | Strong drive toward products |
| At equilibrium | \( \mathrm{Q = K} \) | \( \mathrm{E_{cell} = 0} \) | No net electron flow |
| Near equilibrium (many products) | \( \mathrm{Q > 1} \) | Low \( \mathrm{E_{cell}} \) | Weak drive toward products |
Example:
Consider a galvanic cell represented as \( \mathrm{Zn(s) | Zn^{2+}(aq) || Cu^{2+}(aq) | Cu(s)} \). Under standard conditions, \( \mathrm{E^\circ_{cell} = +1.10\ V} \). Predict qualitatively how \( \mathrm{E_{cell}} \) will change if \( \mathrm{[Cu^{2+}]} \) is increased and \( \mathrm{[Zn^{2+}]} \) is decreased.
▶️ Answer / Explanation
Step 1: For the overall reaction:
\( \mathrm{Zn(s) + Cu^{2+}(aq) \rightarrow Zn^{2+}(aq) + Cu(s)} \)
Step 2: Reaction quotient \( \mathrm{Q = \dfrac{[Zn^{2+}]}{[Cu^{2+}]}} \)
Step 3: Increasing \( \mathrm{[Cu^{2+}]} \) and decreasing \( \mathrm{[Zn^{2+}]} \) → \( \mathrm{Q < 1} \)
Step 4: Since \( \mathrm{Q < K} \), the reaction is more product-favored → larger \( \mathrm{E_{cell}} \)
Final Answer: Increasing \( \mathrm{[Cu^{2+}]} \) and decreasing \( \mathrm{[Zn^{2+}]} \) makes the reaction farther from equilibrium, increasing the cell potential above \( \mathrm{1.10\ V} \).
Why Le Châtelier’s Principle Does Not Apply to Electrochemical Systems
In an electrochemical cell, the system is not at equilibrium — instead, a redox reaction is actively producing or consuming electrical energy. Because of this, Le Châtelier’s Principle (which applies to systems at or near equilibrium) cannot be directly applied to explain changes in cell potential.
Instead of using equilibrium-shift reasoning, changes in cell potential (\( \mathrm{E_{cell}} \)) under nonstandard conditions are better understood using the Nernst equation or qualitative reasoning involving the reaction quotient \( \mathrm{Q} \).
Key Conceptual Difference
| Aspect | Equilibrium System (Le Châtelier’s Principle) | Electrochemical System |
|---|---|---|
| State of the system | At or near equilibrium | Not at equilibrium (ongoing redox reaction) |
| Type of change | Shifts equilibrium position | Alters driving force (cell potential) |
| Governing quantity | Equilibrium constant \( \mathrm{K} \) | Reaction quotient \( \mathrm{Q} \) |
| Response to change | System shifts left or right to reestablish equilibrium | Cell potential (\( \mathrm{E_{cell}} \)) changes, altering reaction spontaneity |
| Final condition | New equilibrium position | System continues until equilibrium (when \( \mathrm{E_{cell} = 0} \)) |
Conceptual Understanding
- Le Châtelier’s principle describes how an equilibrium system shifts when disturbed.
- In contrast, an electrochemical cell is an open system in which continuous redox reactions occur until equilibrium is reached.
- During operation, electron flow and ion migration prevent the establishment of equilibrium; thus, potential differences drive reaction progress rather than equilibrium shifts.
Qualitative Reasoning Instead of Le Châtelier
To predict changes in cell potential due to concentration, pressure, or other factors, use reaction quotient reasoning:
- If \( \mathrm{Q < K} \): system has excess reactants → cell potential increases → more spontaneous.
- If \( \mathrm{Q > K} \): system has excess products → cell potential decreases → less spontaneous.
Example:
The Daniell cell is represented as \( \mathrm{Zn(s) | Zn^{2+}(aq) || Cu^{2+}(aq) | Cu(s)} \), with \( \mathrm{E^\circ_{cell} = +1.10\ V} \). If more \( \mathrm{Cu^{2+}} \) ions are added to the cathode compartment, can Le Châtelier’s principle be used to predict the effect on \( \mathrm{E_{cell}} \)?
▶️ Answer / Explanation
Step 1: The system is not at equilibrium — redox reactions are actively occurring, so Le Châtelier’s principle (which applies to equilibria) cannot be directly applied.
Step 2: Instead, use the reaction quotient \( \mathrm{Q = \dfrac{[Zn^{2+}]}{[Cu^{2+}]}} \).
Adding \( \mathrm{Cu^{2+}} \) decreases \( \mathrm{Q} \), making \( \mathrm{Q < K} \).
Step 3: Qualitative prediction:
- The reaction is further from equilibrium (more reactants relative to products).
- Cell potential increases (\( \mathrm{E_{cell} > E^\circ_{cell}} \)).
Final Answer: Le Châtelier’s principle does not explain the change — but \( \mathrm{E_{cell}} \) increases because the reaction becomes more product-favored as \( \mathrm{Q < K} \).
Relationship Between Cell Potential, \( \mathrm{Q} \), and Equilibrium
The cell potential (\( \mathrm{E_{cell}} \)) of an electrochemical cell represents the driving force for the redox reaction to move toward equilibrium. The magnitude of \( \mathrm{E_{cell}} \) depends on how far the reaction is from equilibrium, as described by the reaction quotient \( \mathrm{Q} \).
At standard conditions (\( \mathrm{Q = 1} \)), the cell potential equals the standard cell potential (\( \mathrm{E^\circ_{cell}} \)). As the system approaches equilibrium, \( \mathrm{E_{cell}} \) decreases in magnitude and becomes zero when \( \mathrm{Q = K} \).
Key Relationships
The relationship between cell potential, concentration, and equilibrium position is qualitatively described by the Nernst equation: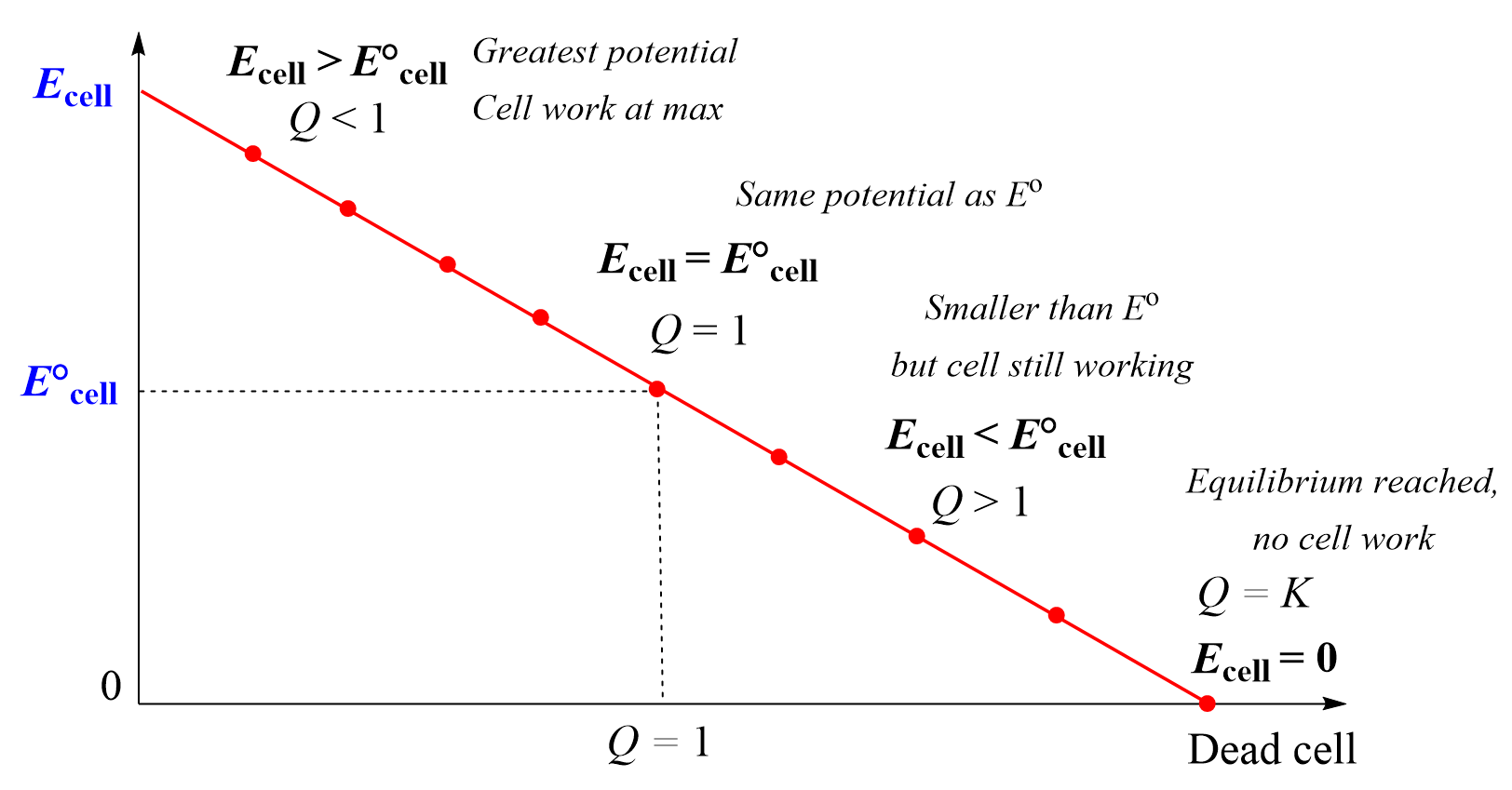
\( \mathrm{E_{cell} = E^\circ_{cell}~ -~ \tfrac{RT}{nF} \ln Q} \)
- \( \mathrm{E_{cell}} \): actual cell potential (V)
- \( \mathrm{E^\circ_{cell}} \): standard cell potential (V)
- \( \mathrm{R} \): universal gas constant = \( \mathrm{8.314\ J\ mol^{-1}\ K^{-1}} \)
- \( \mathrm{T} \): temperature (K)
- \( \mathrm{n} \): moles of electrons transferred
- \( \mathrm{F} \): Faraday’s constant = \( \mathrm{96,485\ C/mol\ e^-} \)
- \( \mathrm{Q} \): reaction quotient = \( \mathrm{\dfrac{[products]^{coeff}}{[reactants]^{coeff}}} \)
Conceptual Behavior of Cell Potential
| Condition | Value of \( \mathrm{Q} \) | Relationship to \( \mathrm{E^\circ_{cell}} \) | Effect on Reaction |
|---|---|---|---|
| At standard conditions | \( \mathrm{Q = 1} \) | \( \mathrm{E_{cell} = E^\circ_{cell}} \) | Reference potential under 1 M, 1 atm conditions |
| System far from equilibrium | \( \mathrm{Q < K} \) | \( \mathrm{E_{cell} > E^\circ_{cell}} \) | Reaction strongly product-favored (forward direction) |
| System near equilibrium | \( \mathrm{Q > 1} \) | \( \mathrm{E_{cell} < E^\circ_{cell}} \) | Reaction weakly product-favored |
| At equilibrium | \( \mathrm{Q = K} \) | \( \mathrm{E_{cell} = 0} \) | No net redox reaction (steady state) |
Conceptual Summary
- \( \mathrm{E_{cell}} \) decreases as the system moves toward equilibrium.
- The reaction is most spontaneous when \( \mathrm{Q} \) is small (many reactants, few products).
- At equilibrium, \( \mathrm{E_{cell}} = 0\) because no further work can be done — the reaction has reached its balance point.
Example:
The redox reaction \( \mathrm{Zn(s) + Cu^{2+}(aq) \rightarrow Zn^{2+}(aq) + Cu(s)} \) has \( \mathrm{E^\circ_{cell} = +1.10\ V} \). Predict qualitatively how the cell potential changes as the reaction proceeds and more \( \mathrm{Zn^{2+}} \) is produced.
▶️ Answer / Explanation
Step 1: Write the reaction quotient:
\( \mathrm{Q = \dfrac{[Zn^{2+}]}{[Cu^{2+}]}} \)
Step 2: As the reaction proceeds, \( \mathrm{[Zn^{2+}]} \) increases and \( \mathrm{[Cu^{2+}]} \) decreases → \( \mathrm{Q} \) increases.
Step 3: According to the Nernst equation, when \( \mathrm{Q > 1} \), \( \mathrm{E_{cell} < E^\circ_{cell}} \).
Step 4: Therefore, as \( \mathrm{Q} \) increases and the system moves toward equilibrium, the cell potential decreases gradually until \( \mathrm{E_{cell} = 0} \) at equilibrium.
Final Answer: As the reaction progresses and products accumulate, \( \mathrm{E_{cell}} \) decreases in magnitude — approaching zero at equilibrium when \( \mathrm{Q = K} \).
Qualitative Understanding of the Nernst Equation and Concentration Effects
The Nernst equation describes how the cell potential (\( \mathrm{E_{cell}} \)) changes under nonstandard conditions (when reactant and product concentrations differ from 1 M). Although the full quantitative form of the equation can be used for calculations, students should understand it qualitatively — how variations in concentration affect the cell’s voltage and spontaneity.
The Nernst Equation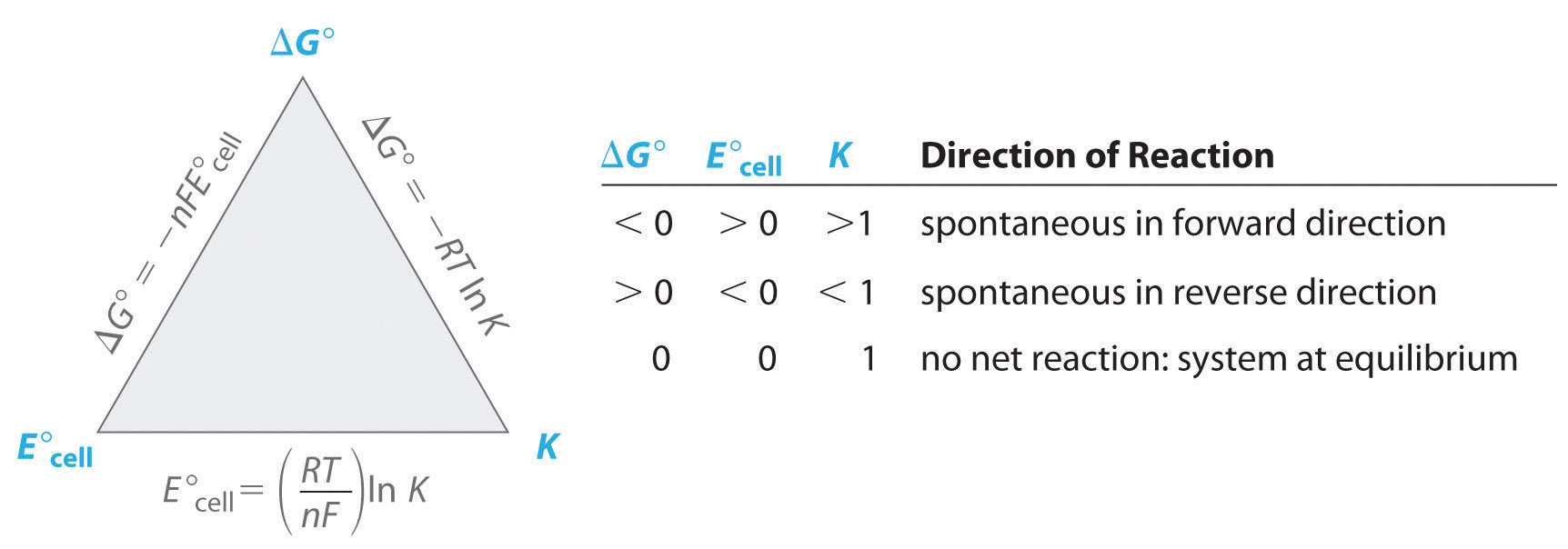
\( \mathrm{E_{cell} = E^\circ_{cell}~ – ~\dfrac{RT}{nF} \ln Q} \)
- \( \mathrm{E_{cell}} \): actual cell potential (V)
- \( \mathrm{E^\circ_{cell}} \): standard cell potential (V)
- \( \mathrm{R = 8.314\ J\ mol^{-1}\ K^{-1}} \): gas constant
- \( \mathrm{T} \): temperature (K)
- \( \mathrm{n} \): moles of electrons transferred
- \( \mathrm{F = 96,485\ C/mol\ e^-} \): Faraday’s constant
- \( \mathrm{Q} \): reaction quotient, \( \mathrm{Q = \dfrac{[Products]^{coeff}}{[Reactants]^{coeff}}} \)
Qualitative Interpretation
- When \( \mathrm{Q < 1} \) (more reactants than products): \( \mathrm{\ln Q} \) is negative → the second term becomes negative, so \( \mathrm{E_{cell} > E^\circ_{cell}} \) → reaction more spontaneous.
- When \( \mathrm{Q = 1} \): \( \mathrm{E_{cell} = E^\circ_{cell}} \)
- When \( \mathrm{Q > 1} \) (more products than reactants): \( \mathrm{\ln Q} \) is positive → the second term reduces the cell potential, so \( \mathrm{E_{cell} < E^\circ_{cell}} \) → reaction less spontaneous.
Conceptual Meaning:
The Nernst equation reflects that the driving force of an electrochemical reaction decreases as the system moves closer to equilibrium. The potential (\( \mathrm{E_{cell}} \)) acts as a measure of how far the reaction is from equilibrium.
Effect of Concentration Changes
| Condition | Effect on \( \mathrm{Q} \) | Effect on \( \mathrm{E_{cell}} \) | Result |
|---|---|---|---|
| Increase in reactant concentration | Decreases \( \mathrm{Q} \) | \( \mathrm{E_{cell}} \) increases | Reaction becomes more spontaneous |
| Increase in product concentration | Increases \( \mathrm{Q} \) | \( \mathrm{E_{cell}} \) decreases | Reaction becomes less spontaneous |
| At equilibrium | \( \mathrm{Q = K} \) | \( \mathrm{E_{cell} = 0} \) | No net reaction |
Example:
A galvanic cell is constructed as \( \mathrm{Zn(s) | Zn^{2+}(aq, 0.10\ M) || Cu^{2+}(aq, 1.0\ M) | Cu(s)} \). The standard cell potential is \( \mathrm{E^\circ_{cell} = +1.10\ V} \). Predict qualitatively whether the actual \( \mathrm{E_{cell}} \) will be greater or smaller than \( \mathrm{E^\circ_{cell}} \).
▶️ Answer / Explanation
Step 1: Write the overall cell reaction:
\( \mathrm{Zn(s) + Cu^{2+}(aq) \rightarrow Zn^{2+}(aq) + Cu(s)} \)
Step 2: Write the reaction quotient:
\( \mathrm{Q = \dfrac{[Zn^{2+}]}{[Cu^{2+}]}} = \dfrac{0.10}{1.0} = 0.10 \)
Step 3: \( \mathrm{Q < 1} \), so \( \ln Q \) is negative. According to the Nernst equation, subtracting a negative term increases \( \mathrm{E_{cell}} \).
Step 4: Therefore, \( \mathrm{E_{cell} > E^\circ_{cell}} \). The cell potential is higher because the reaction is far from equilibrium and strongly product-favored.
Final Answer: \( \mathrm{E_{cell}} \) is greater than \( \mathrm{1.10\ V} \). The cell operates with increased voltage due to higher driving force for electron flow.
