AP Chemistry 8.5 Acid-Base Titrations Study Notes - New Syllabus Effective fall 2024
AP Chemistry 8.5 Acid-Base Titrations Study Notes- New syllabus
AP Chemistry 8.5 Acid-Base Titrations Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain results from the titration of a mono- or polyprotic acid or base solution, in relation to the properties of the solution and its components.
Key Concepts:
- Acid–Base Titration and Titration Curves
- The Equivalence Point and Stoichiometry
- The Half-Equivalence Point and Determination of \( \mathrm{pK_a} \)
- pH at the Equivalence Point
- Titration of Polyprotic Acids
Acid–Base Titration and Titration Curves
A titration is an experimental technique in which an acid–base reaction is carried out under controlled conditions. It involves the gradual addition of one reactant (the titrant) of known concentration to another (the analyte) of unknown concentration until the reaction reaches the equivalence point.
Titration Curve:
The titration curve plots the pH of the solution against the volume of titrant added.
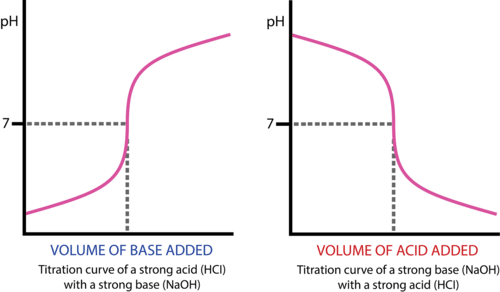
- The curve summarizes how pH changes during the reaction.
- Distinct regions of the curve correspond to the initial solution, buffering region, equivalence point, and excess titrant region.
Key Features of a Typical Titration Curve:
- Initial pH depends on the nature (acidic or basic) of the analyte.
- pH changes slowly near the beginning, then sharply near equivalence.
- The shape differs for strong acid–strong base vs. weak acid–strong base titrations.
Example:
Sketch the general shape of the titration curve for the titration of 25.0 mL of 0.10 M HCl with 0.10 M NaOH.
▶️ Answer / Explanation
- Starts at low pH (strong acid).
- Rises gradually, then sharply near the equivalence point (pH ≈ 7).
- Levels off at high pH once excess base is added.
Final Shape: S-shaped curve crossing pH = 7 at the midpoint.
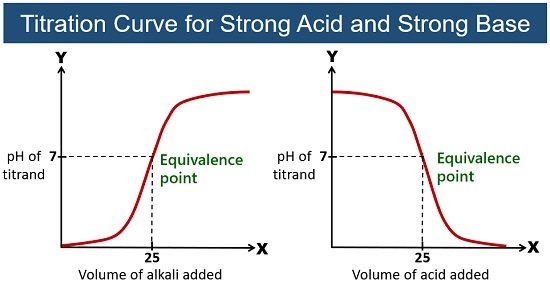
The Equivalence Point and Stoichiometry
The equivalence point occurs when the moles of titrant added equal the moles of analyte present initially. At this point, the acid and base have reacted completely according to their stoichiometric ratio.
Mathematical Relationship:
\( \mathrm{n_{acid} = n_{base}} \)
\( \mathrm{M_{acid}V_{acid} = M_{base}V_{base}} \)
Applications:
- Used to calculate the unknown concentration of an acid or base.
- Applies to strong–strong and weak–strong acid–base titrations.
Example:
25.0 mL of NaOH requires 30.0 mL of 0.100 M HCl for neutralization. What is the molarity of NaOH?
▶️ Answer / Explanation
Step 1: At equivalence: \( \mathrm{M_{acid}V_{acid} = M_{base}V_{base}} \).
Step 2: \( \mathrm{(0.100)(0.0300) = M_{NaOH}(0.0250)} \).
Step 3: \( \mathrm{M_{NaOH} = 0.120\ M} \).
Final Answer: \( \mathrm{[NaOH] = 0.120\ M} \).
The Half-Equivalence Point and Determination of \( \mathrm{pK_a} \)
The half-equivalence point occurs halfway to the equivalence point in a weak acid–strong base titration. At this point, half of the weak acid has been converted to its conjugate base.
Key Relationship: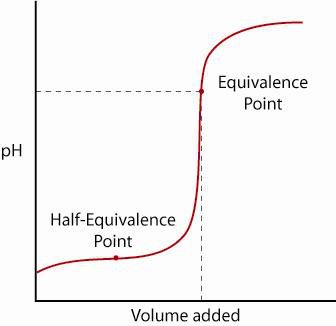
\( \mathrm{[HA] = [A^-]} \)
Substituting into the Henderson–Hasselbalch equation:
\( \mathrm{pH = pK_a + \log\!\left(\dfrac{[A^-]}{[HA]}\right)} \)
Since \( \mathrm{[HA] = [A^-]} \), \( \mathrm{pH = pK_a} \).
Applications:
- The half-equivalence point allows direct experimental determination of \( \mathrm{pK_a} \) from a titration curve.
- Occurs in the buffering region of the titration curve.
Example:
In the titration of acetic acid with NaOH, the pH at the half-equivalence point is 4.76. What is the \( \mathrm{pK_a} \) of acetic acid?
▶️ Answer / Explanation
At half-equivalence: \( \mathrm{pH = pK_a} \).
Final Answer: \( \mathrm{pK_a = 4.76} \).
pH at the Equivalence Point
The pH at the equivalence point depends on the nature of the acid and base involved. At this point, all of one reactant has been neutralized, and the solution contains the conjugate species of the acid or base.
Key Outcomes:
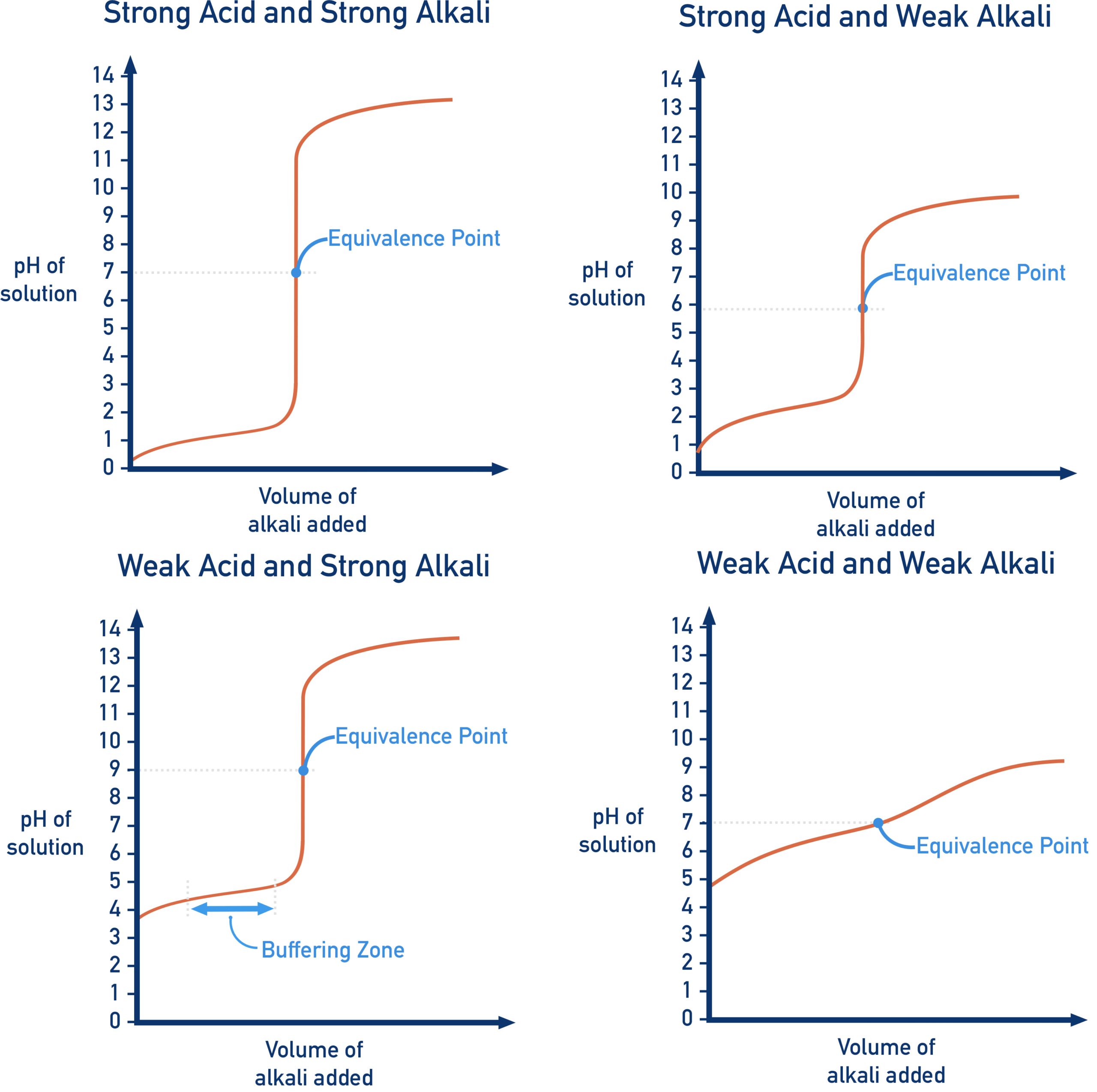
- Strong acid + strong base: neutral solution (pH = 7).
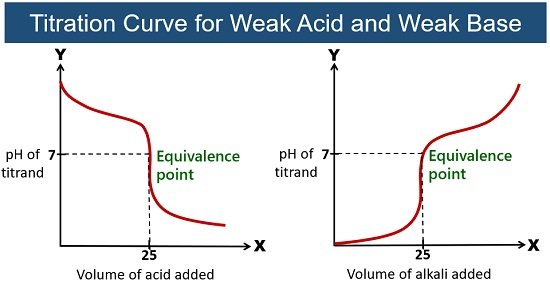
- Weak acid + strong base: conjugate base hydrolyzes → pH > 7 (basic solution).
- Weak base + strong acid: conjugate acid hydrolyzes → pH < 7 (acidic solution).
- Weak acid + weak base: neutral solution (pH = 7).
Example Reactions:
- \( \mathrm{CH_3COOH + NaOH \rightarrow CH_3COONa + H_2O} \) → basic.
- \( \mathrm{NH_3 + HCl \rightarrow NH_4Cl} \) → acidic.
Example:
Predict the pH at the equivalence point for titrating 25.0 mL of 0.10 M NH₃ with 0.10 M HCl.
▶️ Answer / Explanation
At equivalence, solution contains \( \mathrm{NH_4^+} \), a weak acid.
\( \mathrm{NH_4^+ + H_2O \rightleftharpoons NH_3 + H_3O^+} \)
Hydrolysis makes pH < 7.
Final Answer: Solution is acidic (pH ≈ 5.5).
Titration of Polyprotic Acids
Polyprotic acids can donate more than one proton per molecule. Their titration curves display multiple equivalence points — one for each ionizable proton.
Example: \( \mathrm{H_2CO_3} \), \( \mathrm{H_2SO_4} \), \( \mathrm{H_3PO_4} \)
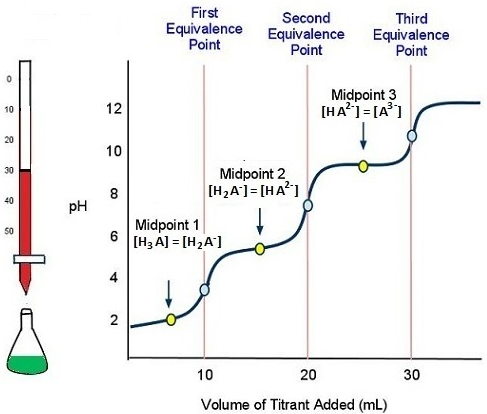
- Each acidic proton dissociates stepwise, with its own \( \mathrm{K_a} \) value.
- The titration curve shows distinct buffering regions and equivalence points for each proton.
Key Relationships:
- First equivalence point → neutralization of first proton.
- Second equivalence point → neutralization of second proton.
- Each half-equivalence point corresponds to a distinct \( \mathrm{pK_a} \).
Example:
Describe the titration curve for \( \mathrm{H_2CO_3} \) with NaOH.
▶️ Answer / Explanation
- Two equivalence points appear on the curve.
- First (pH ≈ 8.3) corresponds to \( \mathrm{H_2CO_3 \rightarrow HCO_3^-} \).
- Second (pH ≈ 10.3) corresponds to \( \mathrm{HCO_3^- \rightarrow CO_3^{2-}} \).
- Each midpoint gives \( \mathrm{pK_{a1}} \) and \( \mathrm{pK_{a2}} \).
