AP Physics 2- 10.1 Electric Charge and Electric Force- Study Notes- New Syllabus
AP Physics 2- 10.1 Electric Charge and Electric Force – Study Notes
AP Physics 2- 10.1 Electric Charge and Electric Force – Study Notes – per latest Syllabus.
Key Concepts:
- Electric Charge
- Coulomb’s Law
- Electrostatic Force: Direction and Applications
- Electric and Gravitational Forces between Charged Objects with Mass
- Electric Permittivity of a Material or Medium
Electric Charge
Electric charge is a fundamental property of matter that gives rise to electric forces and electric fields. Charged particles interact by exerting forces on one another, either attractive or repulsive.
Key Properties of Electric Charge: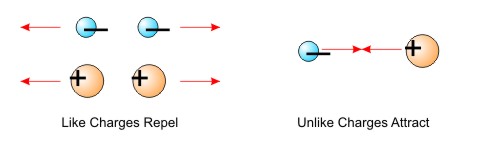
- Two Types: Positive (+) and Negative (−). Like charges repel, unlike charges attract.
- Quantization: Charge exists in discrete multiples of the elementary charge \( e = 1.6 \times 10^{-19} \, C \).
- Conservation of Charge: The net charge in an isolated system remains constant. Charge can neither be created nor destroyed, only transferred.
- Additive Property: Total charge is the algebraic sum of all charges in a system.
- Transfer Mechanisms: Charging can occur by conduction, induction, or friction.
Unit of Charge:
- SI unit: Coulomb (C).
- 1 Coulomb = \( 6.24 \times 10^{18} \) elementary charges.
Relation to Current:
Electric current is the rate of flow of charge:
\( I = \dfrac{Q}{t} \)
- \( I \): Current (A)
- \( Q \): Charge (C)
- \( t \): Time (s)
Example :
A metal sphere carries a charge of \( 3.2 \times 10^{-19} \, C \). How many excess electrons does it have?
▶️ Answer/Explanation
Number of electrons: \( n = \dfrac{Q}{e} \)
\( n = \dfrac{3.2 \times 10^{-19}}{1.6 \times 10^{-19}} = 2 \)
Answer: The sphere has 2 excess electrons.
Example :
A current of 2 A flows through a wire for 5 seconds. Find the total charge transferred.
▶️ Answer/Explanation
\( Q = I \cdot t \)
\( Q = 2 \times 5 = 10 \, C \)
Answer: Total charge transferred = 10 C.
Coulomb’s Law
Coulomb’s Law describes the electrostatic force between two point charges. It is an inverse-square law similar to Newton’s Law of Gravitation, but it can be either attractive or repulsive depending on the signs of the charges.
Mathematical Expression: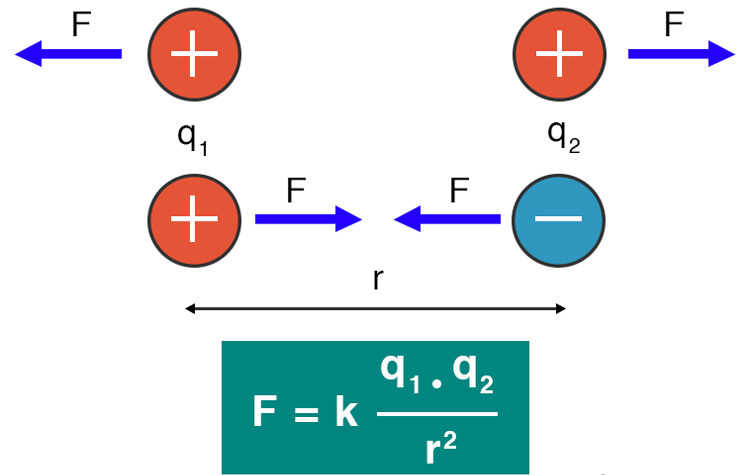
\( F = k_e \dfrac{|q_1 q_2|}{r^2} \)
- \( F \): Magnitude of electrostatic force (N)
- \( q_1, q_2 \): Magnitudes of the two charges (C)
- \( r \): Distance between the charges (m)
- \( k_e \): Coulomb’s constant = \( 8.99 \times 10^9 \, N \, m^2 / C^2 \)
Key Points:
- The force acts along the line joining the two charges.
- Like charges repel, unlike charges attract.
- Coulomb’s law applies strictly to point charges or spherically symmetric charge distributions.
- Superposition Principle: The net force on a charge due to multiple charges is the vector sum of all individual forces.
Example :
Two charges, \( q_1 = +2 \, \mu C \) and \( q_2 = -3 \, \mu C \), are placed 0.5 m apart. Find the magnitude of the electrostatic force between them.
▶️ Answer/Explanation
\( F = k_e \dfrac{|q_1 q_2|}{r^2} \)
\( = (8.99 \times 10^9) \dfrac{(2 \times 10^{-6})(3 \times 10^{-6})}{(0.5)^2} \)
\( = (8.99 \times 10^9) \dfrac{6 \times 10^{-12}}{0.25} \)
\( = (8.99 \times 10^9)(2.4 \times 10^{-11}) \)
\( \approx 0.216 \, N \)
Answer: Force ≈ 0.22 N (attractive).
Example :
Two charges \( q_1 = q_2 = 5 \, \mu C \) are placed 0.1 m apart. Find the electrostatic force between them.
▶️ Answer/Explanation
\( F = k_e \dfrac{|q_1 q_2|}{r^2} \)
\( = (8.99 \times 10^9) \dfrac{(5 \times 10^{-6})(5 \times 10^{-6})}{(0.1)^2} \)
\( = (8.99 \times 10^9) \dfrac{25 \times 10^{-12}}{0.01} \)
\( = (8.99 \times 10^9)(2.5 \times 10^{-9}) \)
\( \approx 22.5 \, N \)
Answer: Force ≈ 22.5 N (repulsive).
Electrostatic Force: Direction and Applications
Direction of Electrostatic Force: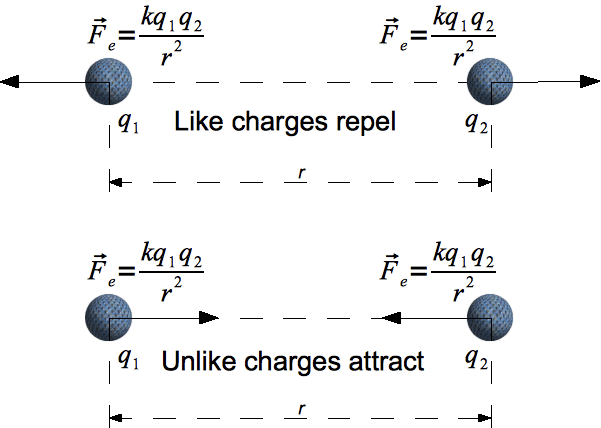
The electrostatic force always acts along the line joining the two charges.
- Like charges repel: Force vectors point away from each other.
- Unlike charges attract: Force vectors point toward each other.
The direction can be determined by considering the sign of charges and applying Coulomb’s law vectorially:
\( \vec{F}_{12} = k_e \dfrac{q_1 q_2}{r^2} \hat{r}_{12} \)
where \( \hat{r}_{12} \) is the unit vector from \( q_1 \) to \( q_2 \).
In systems with multiple charges, the principle of superposition is used → net force is the vector sum of individual forces.
Applications of Electrostatic Force:
- Atomic and Molecular Forces: Binding electrons to nuclei, intermolecular interactions.
- Electrostatic Precipitators: Used in industries to remove dust and smoke particles from exhaust gases by charging particles and attracting them to oppositely charged plates.
- Xerography (Photocopiers): Electrostatic charge is used to attract toner particles onto paper.
- Van de Graaff Generator: Demonstrates accumulation of charge and strong electric forces.
- Biological Systems: Electrostatic interactions stabilize protein structures and DNA.
- Everyday Phenomena: Static electricity, lightning, and balloon-wall attraction after rubbing.
Example :
Two charges \( +3 \, \mu C \) and \( +2 \, \mu C \) are placed 0.4 m apart. Find the force on each charge and its direction.
▶️ Answer/Explanation
\( F = k_e \dfrac{|q_1 q_2|}{r^2} \)
\( = (8.99 \times 10^9) \dfrac{(3 \times 10^{-6})(2 \times 10^{-6})}{(0.4)^2} \)
\( = (8.99 \times 10^9) \dfrac{6 \times 10^{-12}}{0.16} \)
\( = (8.99 \times 10^9)(3.75 \times 10^{-11}) \)
\( \approx 0.337 \, N \)
Answer: Force on each charge = 0.34 N, directed away from the other (repulsive).
Example :
Two charges \( +5 \, \mu C \) and \( -2 \, \mu C \) are placed 0.2 m apart. Find the force on each charge and its direction.
▶️ Answer/Explanation
\( F = k_e \dfrac{|q_1 q_2|}{r^2} \)
\( = (8.99 \times 10^9) \dfrac{(5 \times 10^{-6})(2 \times 10^{-6})}{(0.2)^2} \)
\( = (8.99 \times 10^9) \dfrac{10 \times 10^{-12}}{0.04} \)
\( = (8.99 \times 10^9)(2.5 \times 10^{-10}) \)
\( \approx 2.25 \, N \)
Answer: Force on each charge = 2.25 N, directed toward the other (attractive).
Electric and Gravitational Forces between Charged Objects with Mass
When two objects have both charge and mass, they exert two kinds of fundamental forces on each other simultaneously: the electrostatic force and the gravitational force.
Electrostatic Force (Coulomb’s Law):
\( F_e = k_e \dfrac{|q_1 q_2|}{r^2} \)
- Always acts along the line joining the two charges.
- Repulsive for like charges, attractive for unlike charges.
- Typically much stronger than gravitational force for elementary particles.
Gravitational Force (Newton’s Law of Gravitation):
\( F_g = G \dfrac{m_1 m_2}{r^2} \)
- Always attractive in nature.
- Acts along the line joining the two masses.
- Magnitude depends on the product of masses and inverse square of distance.
Comparison of Forces:
- Electrostatic force can be attractive or repulsive, but gravitational force is always attractive.
- For small particles like protons/electrons, the electric force is enormously stronger than the gravitational force.
- For massive neutral bodies (like planets), gravitational forces dominate because the net charge is nearly zero.
Example :
Find the ratio of electrostatic force to gravitational force between two protons separated by \( r = 1.0 \times 10^{-15} \, m \).
▶️ Answer/Explanation
Electrostatic Force:
\( F_e = k_e \dfrac{e^2}{r^2} = (8.99 \times 10^9) \dfrac{(1.6 \times 10^{-19})^2}{(1.0 \times 10^{-15})^2} \)
\( = (8.99 \times 10^9)(2.56 \times 10^{-38})/(1.0 \times 10^{-30}) \)
\( = 2.3 \times 10^{2} \, N \)
Gravitational Force:
\( F_g = G \dfrac{m_p^2}{r^2} = (6.67 \times 10^{-11}) \dfrac{(1.67 \times 10^{-27})^2}{(1.0 \times 10^{-15})^2} \)
\( = (6.67 \times 10^{-11})(2.79 \times 10^{-54})/(1.0 \times 10^{-30}) \)
\( = 1.86 \times 10^{-34} \, N \)
Ratio: \( \dfrac{F_e}{F_g} \approx \dfrac{2.3 \times 10^{2}}{1.86 \times 10^{-34}} \approx 1.2 \times 10^{36} \)
Answer: Electrostatic force is about \( 10^{36} \) times stronger than gravitational force between two protons.
Electric Permittivity of a Material or Medium
Electric permittivity is a property of a material that describes how much it opposes the formation of an electric field within it. It determines how a material affects the interaction between electric charges when placed in that medium.
Absolute Permittivity (\( \varepsilon \)):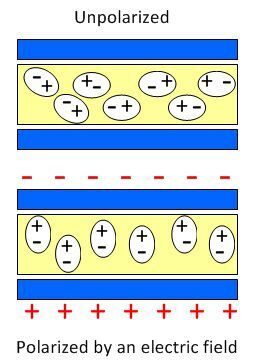
- Symbol: \( \varepsilon \)
- Relates electric displacement field (\( \vec{D} \)) to electric field (\( \vec{E} \)):
\( \vec{D} = \varepsilon \vec{E} \)
- Unit: \( C^2 N^{-1} m^{-2} \) or \( F/m \) (farads per meter).
- In vacuum, permittivity is a constant:
\( \varepsilon_0 = 8.85 \times 10^{-12} \, F/m \)
Relative Permittivity (Dielectric Constant):
Defined as the ratio of permittivity of material to permittivity of free space:
\( \varepsilon_r = \dfrac{\varepsilon}{\varepsilon_0} \)
- Dimensionless quantity.
- Represents how much a medium reduces the effective electric field compared to vacuum.
Effect on Coulomb’s Law:
In a medium, Coulomb’s force between two charges is reduced as:
\( F = \dfrac{1}{4\pi \varepsilon} \dfrac{q_1 q_2}{r^2} = \dfrac{1}{4\pi \varepsilon_0 \varepsilon_r} \dfrac{q_1 q_2}{r^2} \)
- Larger \( \varepsilon_r \) → weaker force between charges.
- Explains why electric interactions in water (high \( \varepsilon_r \)) are weaker compared to vacuum.
Applications of Permittivity:
- Design of capacitors (capacitance increases with higher \( \varepsilon_r \)).
- Propagation of electromagnetic waves in different media.
- Insulating materials in circuits (dielectrics).
- Explains polarization effects inside materials.
Example :
Two charges of \( +2 \, \mu C \) and \( -2 \, \mu C \) are separated by 0.5 m in vacuum. Find the force between them if the space between is filled with a dielectric of relative permittivity \( \varepsilon_r = 5 \).
▶️ Answer/Explanation
Step 1: Force in vacuum
\( F_0 = \dfrac{1}{4\pi \varepsilon_0} \dfrac{q_1 q_2}{r^2} \)
\( = (9 \times 10^9) \dfrac{(2 \times 10^{-6})(2 \times 10^{-6})}{(0.5)^2} \)
\( = (9 \times 10^9)(4 \times 10^{-12})/0.25 \)
\( = 0.144 \, N \)
Step 2: Force in dielectric
\( F = \dfrac{F_0}{\varepsilon_r} = \dfrac{0.144}{5} = 0.0288 \, N \)
Answer: Force ≈ \( 0.029 \, N \) (attractive).
