AP Physics 2- 15.2 The Bohr Model of Atomic Structure- Study Notes- New Syllabus
AP Physics 2- 15.2 The Bohr Model of Atomic Structure – Study Notes
AP Physics 2- 15.2 The Bohr Model of Atomic Structure – Study Notes – per latest Syllabus.
Key Concepts:
- Properties of an Atom
- The Bohr Model of the Atom
Properties of an Atom
An atom is the fundamental building block of matter. It consists of a dense central nucleus (protons + neutrons) surrounded by electrons in quantized energy levels. The structure and properties of an atom determine the chemical behavior of an element.
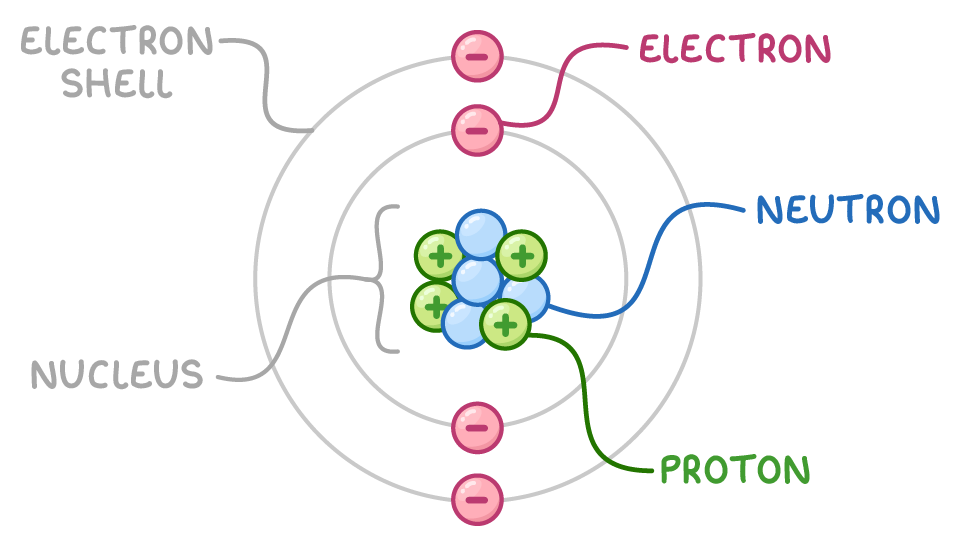
Subatomic Particles:
| Particle | Charge | Mass / Notes |
| \(\mathrm{p^+}\) | \(\mathrm{+1.6 \times 10^{-19} \, C}\) | Mass ≈ \(\mathrm{1.67 \times 10^{-27} \, kg}\), defines atomic number (\(Z\)) |
| \(\mathrm{n^0}\) | 0 | Mass ≈ \(\mathrm{1.67 \times 10^{-27} \, kg}\), variation gives rise to isotopes |
| \(\mathrm{e^-}\) | \(\mathrm{-1.6 \times 10^{-19} \, C}\) | Mass ≈ \(\mathrm{9.11 \times 10^{-31} \, kg}\), arranged in energy levels around the nucleus |
Key Atomic Properties: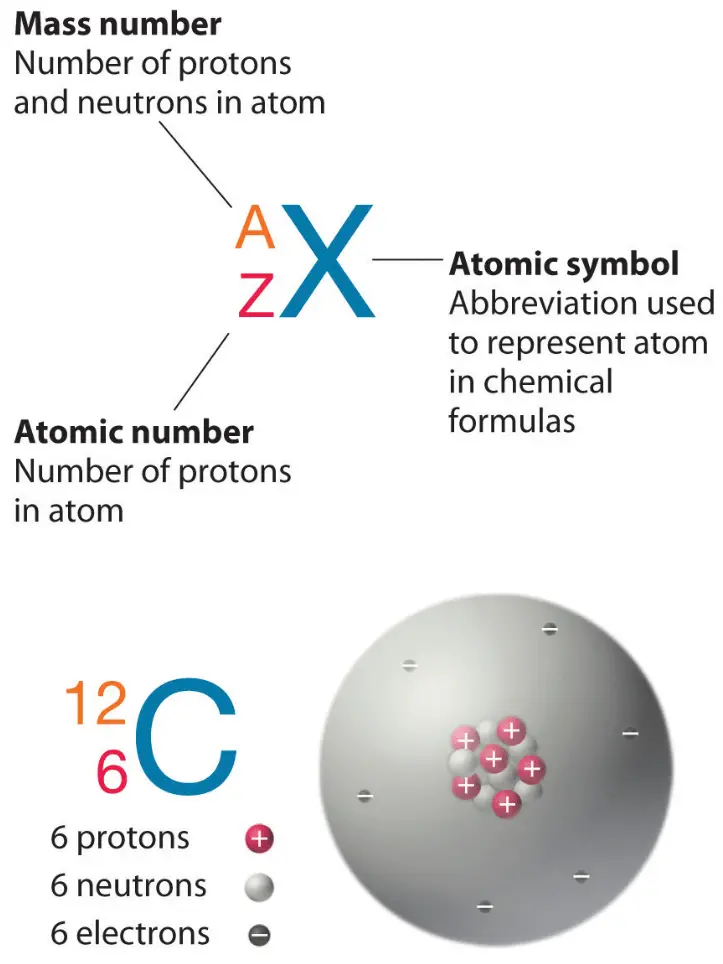
- Atomic Number (\(Z\)): Number of protons in nucleus (defines element).
- Mass Number (\(A\)): \(A = \mathrm{protons + neutrons}\).
- Isotopes: Same \(Z\), different \(A\).
- Electronic Configuration: Distribution of electrons in shells and subshells.
- Charge Neutrality: Stable atom → number of protons = number of electrons.
Size and Scale:
Atomic radius ≈ \(\mathrm{10^{-10} \, m}\) (0.1 nm)
Nucleus radius ≈ \(\mathrm{10^{-15} \, m}\) → most of the atom is empty space.
Quantum Properties:
Electrons show wave-particle duality. Their arrangement follows the Pauli Exclusion Principle, Hund’s Rule, and the Aufbau Principle. Energy levels are quantized, explaining atomic spectra.
Example :
Find the number of protons, neutrons, and electrons in a neutral atom of Carbon-14.
▶️ Answer/Explanation
Step 1: Atomic number (\(Z\)): Carbon has \(Z = 6\) → 6 protons.
Step 2: Mass number (\(A\)): 14 → Neutrons = \(A – Z = 14 – 6 = 8\).
Step 3: Electrons: Neutral atom → 6 electrons.
Final Answer: 6 protons, 8 neutrons, 6 electrons.
Example :
The radius of a hydrogen atom is about \(\mathrm{0.53 \, \text{Å}}\). Estimate how many times larger the atom is compared to its nucleus (\(\mathrm{\sim 1 \, fm}\)).
▶️ Answer/Explanation
Step 1: Atomic radius: \(\mathrm{r_{atom} = 0.53 \times 10^{-10} \, m}\).
Step 2: Nuclear radius: \(\mathrm{r_{nucleus} \approx 1 \times 10^{-15} \, m}\).
Step 3: Ratio: \(\mathrm{\dfrac{r_{atom}}{r_{nucleus}} = \dfrac{0.53 \times 10^{-10}}{1 \times 10^{-15}} \approx 5.3 \times 10^{4}}\).
Final Answer: The atom is about 50,000 times larger than its nucleus → most of it is empty space.
The Bohr Model of the Atom
The Bohr model (1913) explains the stability of atoms and discrete spectral lines, particularly for hydrogen. It combines classical circular orbits with quantum restrictions.
Physical Idea: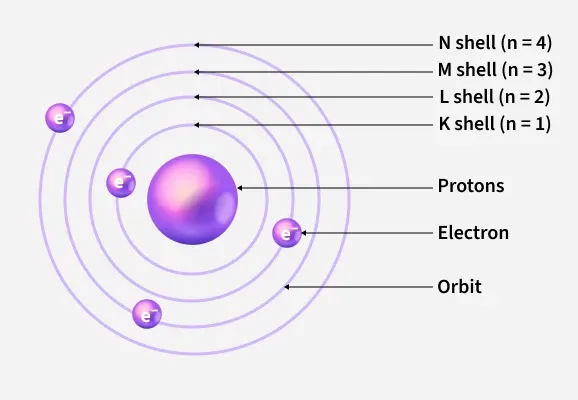
In this model, electrons move in circular orbits around the nucleus. The motion is governed by the Coulomb force of attraction between the electron and the nucleus:
$ \mathrm{F = \dfrac{k e^2}{r^2}} $ where \(\mathrm{k}\) is Coulomb’s constant, \(e\) is the electron charge, and \(r\) is the orbit radius.
This force provides the necessary centripetal acceleration:
$ \mathrm{\dfrac{m_e v^2}{r} = \dfrac{k e^2}{r^2}} $ where \(\mathrm{m_e}\) is the electron mass and \(\mathrm{v}\) is its orbital speed.
Bohr’s Quantization Condition: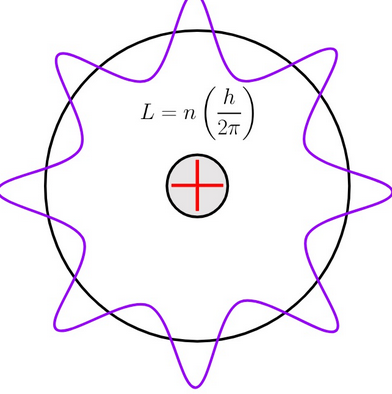
Only orbits where the electron’s angular momentum is quantized are allowed:
$ \mathrm{m_e v r = n \hbar}, \quad n = 1, 2, 3, \dots $ with \(\hbar = \dfrac{h}{2 \pi}\).
Bohr Radius and Electron Velocity:
Solving Coulomb + quantization equations, the radius of the \(n\)-th orbit is:
$ \mathrm{r_n = n^2 a_0}, \quad \mathrm{a_0 = \dfrac{\hbar^2}{k e^2 m_e} \approx 5.29 \times 10^{-11} \, m} $
The velocity of the electron in the \(n\)-th orbit: $ \mathrm{v_n = \dfrac{k e^2}{\hbar} \cdot \dfrac{1}{n} \approx \dfrac{2.18 \times 10^6}{n} \, m/s} $
The total energy of the electron: $ \mathrm{E_n = – \dfrac{k e^2}{2 r_n} = – \dfrac{13.6 \, \text{eV}}{n^2}} $
Key Features:
- Electron orbits are stable and quantized.
- Energy is emitted or absorbed only when electrons jump between orbits: \(\mathrm{\Delta E = E_{final} – E_{initial} = h \nu}\).
- Works well for hydrogen and hydrogen-like ions.
Example :
Find the radius and velocity of an electron in the 2nd orbit (\(n=2\)) of a hydrogen atom.
▶️ Answer/Explanation
Step 1: Radius: \(\mathrm{r_2 = n^2 a_0 = 2^2 \times 5.29 \times 10^{-11} \approx 2.12 \times 10^{-10} \, m}\)
Step 2: Velocity: \(\mathrm{v_2 = \dfrac{2.18 \times 10^6}{2} \approx 1.09 \times 10^6 \, m/s}\)
Example :
Calculate the energy of an electron in the 3rd orbit (\(n=3\)) and the wavelength of a photon emitted when it drops to \(n=2\).
▶️ Answer/Explanation
Step 1: Energy of \(n=3\): \(\mathrm{E_3 = – \dfrac{13.6}{3^2} \approx -1.51 \, eV}\)
Step 2: Energy of \(n=2\): \(\mathrm{E_2 = – \dfrac{13.6}{2^2} = -3.4 \, eV}\)
Step 3: Energy of photon: \(\mathrm{\Delta E = E_2 – E_3 = -3.4 – (-1.51) = -1.89 \, eV}\)
Step 4: Wavelength: \(\mathrm{\lambda = \dfrac{hc}{|\Delta E|} = \dfrac{6.63 \times 10^{-34} \cdot 3 \times 10^8}{1.89 \times 1.6 \times 10^{-19}} \approx 656 \, nm}\)
