AP Physics 2- 15.3 Emission and Absorption Spectra- Study Notes- New Syllabus
AP Physics 2- 15.3 Emission and Absorption Spectra – Study Notes
AP Physics 2- 15.3 Emission and Absorption Spectra – Study Notes – per latest Syllabus.
Key Concepts:
- Emission and Absorption of Photons by Atoms
- Quantized Energy Transfer
- Atomic Spectra and Binding Energy
Emission and Absorption of Photons by Atoms
Atoms can absorb or emit energy in the form of photons when electrons transition between energy levels. These processes are quantized, meaning only specific amounts of energy can be absorbed or emitted.
Photon Absorption: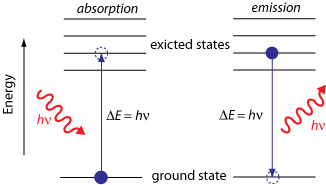
When an electron absorbs a photon of energy \(\mathrm{h \nu}\), it jumps from a lower energy level (\(E_{initial}\)) to a higher energy level (\(E_{final}\)):
$ \mathrm{\Delta E = E_{final} – E_{initial} = h \nu} $
Photon Emission:
When an electron drops from a higher energy level to a lower one, a photon is emitted with energy equal to the difference between the two levels:
$ \mathrm{\Delta E = E_{initial} – E_{final} = h \nu} $
Each transition corresponds to a photon of a single frequency (\(\nu\)) and wavelength (\(\lambda = \dfrac{c}{\nu}\)).
Key Features:
- Photon absorption occurs only if the photon’s energy matches the energy difference between two allowed levels.
- Emission produces discrete spectral lines; the set of lines is unique for each element.
- Ground-state electrons require the maximum energy for ionization; excited electrons require less.
Example :
Determine the wavelength of a photon emitted when an electron in a hydrogen atom drops from \(n=3\) to \(n=1\).
▶️ Answer/Explanation
Step 1: Energy of \(n=3\): \(\mathrm{E_3 = – \dfrac{13.6}{3^2} \approx -1.51 \, eV}\)
Step 2: Energy of \(n=1\): \(\mathrm{E_1 = – 13.6 \, eV}\)
Step 3: Photon energy: \(\mathrm{\Delta E = E_1 – E_3 = -13.6 – (-1.51) = -12.09 \, eV}\)
Step 4: Wavelength: \(\mathrm{\lambda = \dfrac{hc}{|\Delta E|} = \dfrac{6.63 \times 10^{-34} \cdot 3 \times 10^8}{12.09 \cdot 1.6 \times 10^{-19}} \approx 102.6 \, nm}\)
Example :
Calculate the wavelength of a photon absorbed when an electron in a hydrogen atom jumps from \(n=2\) to \(n=4\).
▶️ Answer/Explanation
Step 1: Energy of \(n=2\): \(\mathrm{E_2 = – \dfrac{13.6}{2^2} = -3.4 \, eV}\)
Step 2: Energy of \(n=4\): \(\mathrm{E_4 = – \dfrac{13.6}{4^2} = -0.85 \, eV}\)
Step 3: Energy of photon: \(\mathrm{\Delta E = E_4 – E_2 = -0.85 – (-3.4) = 2.55 \, eV}\)
Step 4: Wavelength: \(\mathrm{\lambda = \dfrac{hc}{\Delta E} = \dfrac{6.63 \times 10^{-34} \cdot 3 \times 10^8}{2.55 \cdot 1.6 \times 10^{-19}} \approx 486 \, nm}\)
Step 5: Interpretation: A photon with wavelength 486 nm (visible blue light) is absorbed to excite the electron from \(n=2\) to \(n=4\).
Quantized Energy Transfer
Energy can only be absorbed or emitted by an atom if the amount of energy matches the difference between two allowed atomic energy states.
Physical Idea:
Electrons occupy discrete energy levels in an atom. A transition between two energy levels occurs only if the photon energy equals the energy difference:
$ \mathrm{\Delta E = E_{final} – E_{initial} = h \nu = \dfrac{hc}{\lambda}}$
where \(\mathrm{h}\) is Planck’s constant, \(\nu\) is the frequency, and \(\lambda\) is the wavelength of the photon.
This means that each transition corresponds to the emission or absorption of a photon of a single frequency and a single wavelength.
Key Features:
- Energy transitions are quantized; not all photon energies are allowed.
- Each spectral line corresponds to a specific electronic transition.
- This explains why atomic spectra consist of discrete lines rather than a continuous spectrum.
Example :
Find the wavelength of a photon emitted when an electron in a hydrogen atom drops from the 4th orbit (\(n=4\)) to the 2nd orbit (\(n=2\)).
▶️ Answer/Explanation
Step 1: Energy of \(n=4\): \(\mathrm{E_4 = – \dfrac{13.6}{4^2} = -0.85 \, eV}\)
Step 2: Energy of \(n=2\): \(\mathrm{E_2 = – \dfrac{13.6}{2^2} = -3.4 \, eV}\)
Step 3: Energy of photon: \(\mathrm{\Delta E = E_2 – E_4 = -3.4 – (-0.85) = -2.55 \, eV}\)
Step 4: Wavelength: \(\mathrm{\lambda = \dfrac{hc}{|\Delta E|} = \dfrac{6.63 \times 10^{-34} \cdot 3 \times 10^8}{2.55 \cdot 1.6 \times 10^{-19}} \approx 486 \, nm}\)
Atomic Spectra and Binding Energy
Atoms of each element have a unique set of allowed energy levels. This gives rise to a unique set of absorption and emission frequencies, which determines the element’s spectrum.
Physical Idea:
- Each element has distinct energy levels due to its specific nuclear charge and electron configuration.
- Transitions between these levels produce photons of specific frequencies (\(\nu\)) and wavelengths (\(\lambda\)), giving the element a unique spectral signature.
- The binding energy is the energy required to remove an electron from an atom, thereby ionizing it.
- An electron in the lowest energy level (ground state) requires the maximum binding energy for removal.
Key Features:
- Each element has a characteristic spectrum that can be used for identification (atomic fingerprint).
- Ionization energy is highest for electrons in the ground state and decreases for higher excited states.
- Photon absorption or emission only occurs if the photon energy matches the energy difference between two allowed levels.
Example :
Calculate the ionization energy of a hydrogen atom in its ground state.
▶️ Answer/Explanation
Step 1: Energy of electron in ground state: \(\mathrm{E_1 = – 13.6 \, eV}\)
Step 2: Ionization energy: The energy required to remove the electron is the positive of the ground state energy: \(\mathrm{E_{ion} = |E_1| = 13.6 \, eV}\)
Step 3: Interpretation: The electron must absorb a photon with energy at least 13.6 eV to be ionized from the ground state.
