AP Physics 2- 15.5 The Photoelectric Effect- Study Notes- New Syllabus
AP Physics 2- 15.5 The Photoelectric Effect – Study Notes
AP Physics 2- 15.5 The Photoelectric Effect – Study Notes – per latest Syllabus.
Key Concepts:
- The Photoelectric Effect
- Stopping Potential
The Photoelectric Effect
The photoelectric effect demonstrates the interaction between photons and matter. When light shines on a metal surface, it can eject electrons from the surface if the photons have sufficient energy. This phenomenon provides evidence for the particle nature of light.
Concept:
Photons are particles of light, each with energy: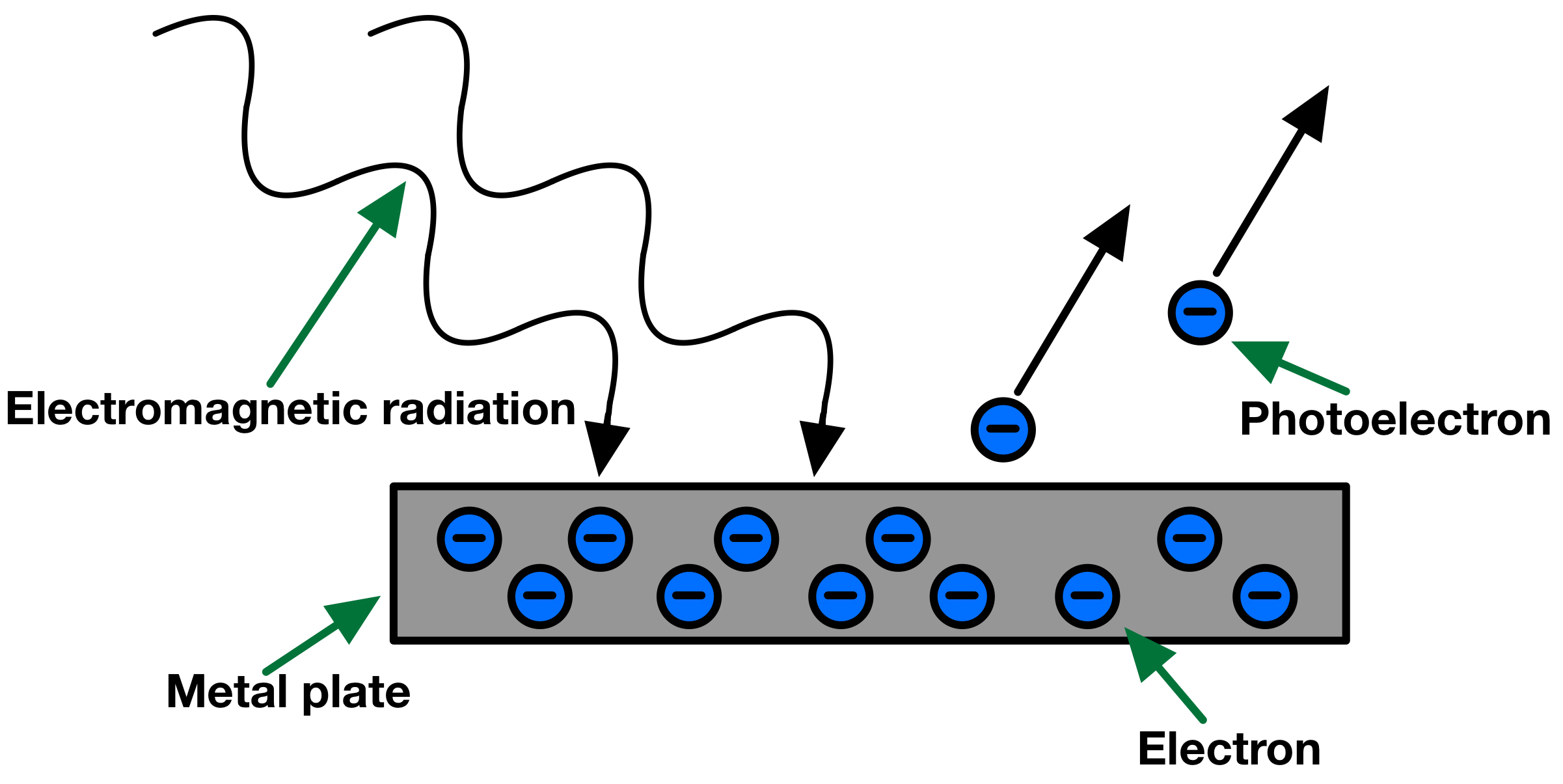
$ \mathrm{E_{photon} = h \nu} $ where \(\mathrm{h}\) is Planck’s constant and \(\mathrm{\nu}\) is the frequency of light.
When a photon strikes a metal, it can transfer its energy to an electron. If the photon energy exceeds the work function (\(\mathrm{\phi}\)) of the metal, the electron is emitted.
The maximum kinetic energy of the emitted electron is:
$ \mathrm{K_{max} = h \nu – \phi} $
No electrons are emitted if \(\mathrm{h \nu < \phi}\), regardless of light intensity.
Work Function
The work function \(\mathrm{\phi}\) is the minimum energy required to remove an electron from the surface of a metal.
- It depends on the type of metal: different metals have different \(\mathrm{\phi}\) values.
- Increasing light intensity increases the number of emitted electrons, but not their kinetic energy.
Key Features
- Electron emission occurs only if photon energy exceeds the work function.
- Photon frequency determines electron emission, not light intensity.
- Maximum kinetic energy of emitted electrons increases with photon frequency.
- Demonstrates the particle-like behavior of light (quantization of energy).
Example :
A metal has a work function \(\mathrm{\phi = 2.0 \, eV}\). Calculate the maximum kinetic energy of an electron emitted when light of frequency \(\mathrm{\nu = 1.0 \times 10^{15} \, Hz}\) shines on it.
▶️ Answer/Explanation
Step 1: Photon energy: \(\mathrm{E_{photon} = h \nu = 6.63 \times 10^{-34} \cdot 1.0 \times 10^{15} \approx 6.63 \times 10^{-19} \, J}\)
Step 2: Convert photon energy to eV: \(\mathrm{E_{photon} = \dfrac{6.63 \times 10^{-19}}{1.6 \times 10^{-19}} \approx 4.14 \, eV}\)
Step 3: Maximum kinetic energy: \(\mathrm{K_{max} = E_{photon} – \phi = 4.14 – 2.0 \approx 2.14 \, eV}\)
Example :
Determine the threshold frequency (\(\mathrm{\nu_0}\)) for the same metal with \(\mathrm{\phi = 2.0 \, eV}\).
▶️ Answer/Explanation
Step 1: Convert work function to joules: \(\mathrm{\phi = 2.0 \cdot 1.6 \times 10^{-19} \approx 3.2 \times 10^{-19} \, J}\)
Step 2: Threshold frequency: \(\mathrm{\nu_0 = \dfrac{\phi}{h} = \dfrac{3.2 \times 10^{-19}}{6.63 \times 10^{-34}} \approx 4.83 \times 10^{14} \, Hz}\)
Step 3: Interpretation: Light with frequency below \(\mathrm{4.83 \times 10^{14} \, Hz}\) cannot eject electrons from this metal.
Stopping Potential
The stopping potential is used to measure the maximum kinetic energy of photoelectrons emitted from a metal when light shines on it. It provides a direct experimental method to verify the photoelectric effect and the particle nature of light.
When photoelectrons are emitted, they can be stopped by applying a reverse (retarding) potential \(\mathrm{V_s}\) that prevents them from reaching the anode. The stopping potential is defined such that the maximum kinetic energy of the electrons is exactly canceled by the electric potential energy: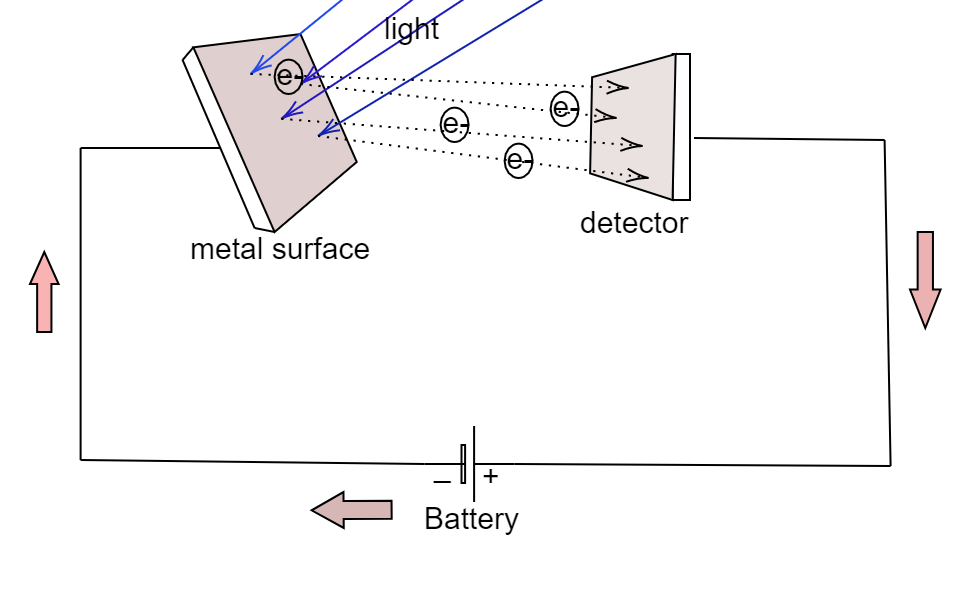
$ \mathrm{K_{max} = e V_s} $ where \(\mathrm{e}\) is the elementary charge.
Combining with the photoelectric equation:
$ \mathrm{K_{max} = h \nu – \phi = e V_s} $
Measurement of \(\mathrm{V_s}\) allows determination of \(\mathrm{h}\), \(\phi\), or the photon energy.
Key Features
- The stopping potential is independent of light intensity; it depends only on photon frequency.
- Maximum kinetic energy of emitted electrons is directly proportional to the stopping potential.
- Verifies the linear relationship between photon frequency and electron kinetic energy.
- Threshold frequency corresponds to \(\mathrm{V_s = 0}\).
Example :
Light of frequency \(\mathrm{\nu = 1.2 \times 10^{15} \, Hz}\) falls on a metal with work function \(\mathrm{\phi = 2.0 \, eV}\). Find the stopping potential.
▶️ Answer/Explanation
Step 1: Photon energy: \(\mathrm{E_{photon} = h \nu = 6.63 \times 10^{-34} \cdot 1.2 \times 10^{15} \approx 7.96 \times 10^{-19} \, J}\)
Step 2: Convert to eV: \(\mathrm{E_{photon} \approx 4.97 \, eV}\)
Step 3: Maximum kinetic energy: \(\mathrm{K_{max} = E_{photon} – \phi = 4.97 – 2.0 \approx 2.97 \, eV}\)
Step 4: Stopping potential: \(\mathrm{V_s = \dfrac{K_{max}}{e} = 2.97 \, V}\)
Example :
Determine the stopping potential for light of frequency \(\mathrm{\nu = 5.0 \times 10^{14} \, Hz}\) on the same metal (\(\mathrm{\phi = 2.0 \, eV}\)).
▶️ Answer/Explanation
Step 1: Photon energy: \(\mathrm{E_{photon} = h \nu = 6.63 \times 10^{-34} \cdot 5.0 \times 10^{14} \approx 3.32 \times 10^{-19} \, J}\)
Step 2: Convert to eV: \(\mathrm{E_{photon} \approx 2.07 \, eV}\)
Step 3: Maximum kinetic energy: \(\mathrm{K_{max} = E_{photon} – \phi = 2.07 – 2.0 \approx 0.07 \, eV}\)
Step 4: Stopping potential: \(\mathrm{V_s = K_{max}/e \approx 0.07 \, V}\)
