AP Physics 2- 9.1 Kinetic Theory of Temperature and Pressure - Study Notes- New Syllabus
AP Physics 2- 9.1 Kinetic Theory of Temperature and Pressure – Study Notes
AP Physics 2- 9.1 Kinetic Theory of Temperature and Pressure – Study Notes – per latest Syllabus.
Key Concepts:
- Pressure of a Gas Due to Atomic Collisions
- Temperature and Atomic Motion
- Maxwell–Boltzmann Distribution
- Root-Mean-Square (RMS) Speed
Pressure of a Gas Due to Atomic Collisions
Gas pressure originates from the microscopic collisions of atoms or molecules with each other and with the walls of the container. These collisions transfer momentum and exert forces, which averaged over time and area, appear as a measurable pressure.
Role of Collisions:
- Atom–Atom Collisions: Gas molecules constantly collide elastically with each other, conserving momentum and energy.
- Atom–Wall Collisions: When a gas atom collides with the container wall, its velocity component perpendicular to the wall changes direction, creating a change in momentum.
- By Newton’s third law, the wall exerts an equal and opposite force on the atom; hence, the atom exerts a force on the wall.
Pressure from Microscopic Forces: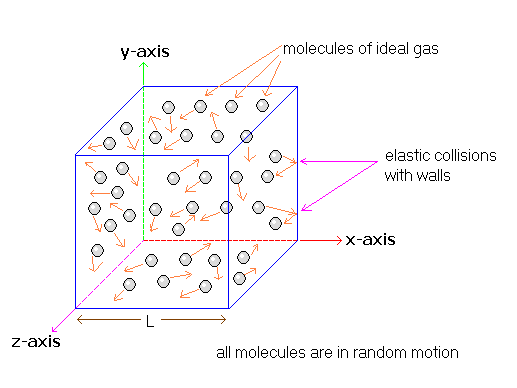
The pressure on a surface is the average effect of these molecular forces:
\( P = \dfrac{\sum F_{\perp}}{A} \)
- \( \sum F_{\perp} \): Sum of perpendicular force components from atoms colliding with the surface
- \( A \): Area of the surface
Thus, pressure is the ratio of total perpendicular force exerted by atomic collisions to the area of contact.
Conservation of Momentum in Collisions:
- Each atom’s change in momentum upon collision determines the impulse delivered to the wall.
- Since collisions are very frequent, the time-averaged rate of momentum transfer is steady, producing uniform pressure.
Pressure Within the Gas:
- Pressure is not only exerted on the container walls, but also exists throughout the fluid.
- This is because molecules collide in all directions, transferring forces equally in the bulk of the gas.
- Thus, every part of the gas, even deep inside, experiences the same pressure (if the gas is in equilibrium).
Example:
A gas atom of mass \( m = 5 \times 10^{-26} \, \text{kg} \) collides elastically with a container wall, reversing its velocity from \( +500 \, \text{m/s} \) to \( -500 \, \text{m/s} \). Find the change in momentum imparted to the wall.
▶️Answer/Explanation
Initial momentum: \( p_i = m v = (5 \times 10^{-26})(+500) = 2.5 \times 10^{-23} \, \text{kg·m/s} \)
Final momentum: \( p_f = m v = (5 \times 10^{-26})(-500) = -2.5 \times 10^{-23} \, \text{kg·m/s} \)
Change in momentum: \( \Delta p = p_f – p_i = -2.5 \times 10^{-23} – 2.5 \times 10^{-23} = -5 \times 10^{-23} \, \text{kg·m/s} \)
Impulse delivered to wall = \( +5 \times 10^{-23} \, \text{kg·m/s} \).
Temperature and Atomic Motion:
The temperature of a system is characterized by the average kinetic energy of the atoms or molecules within it.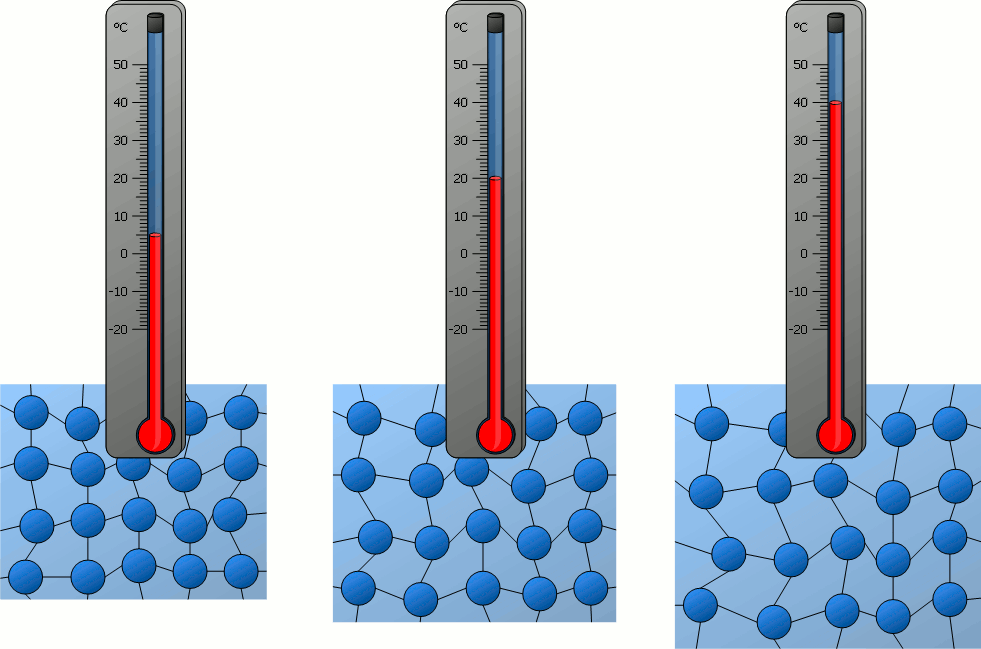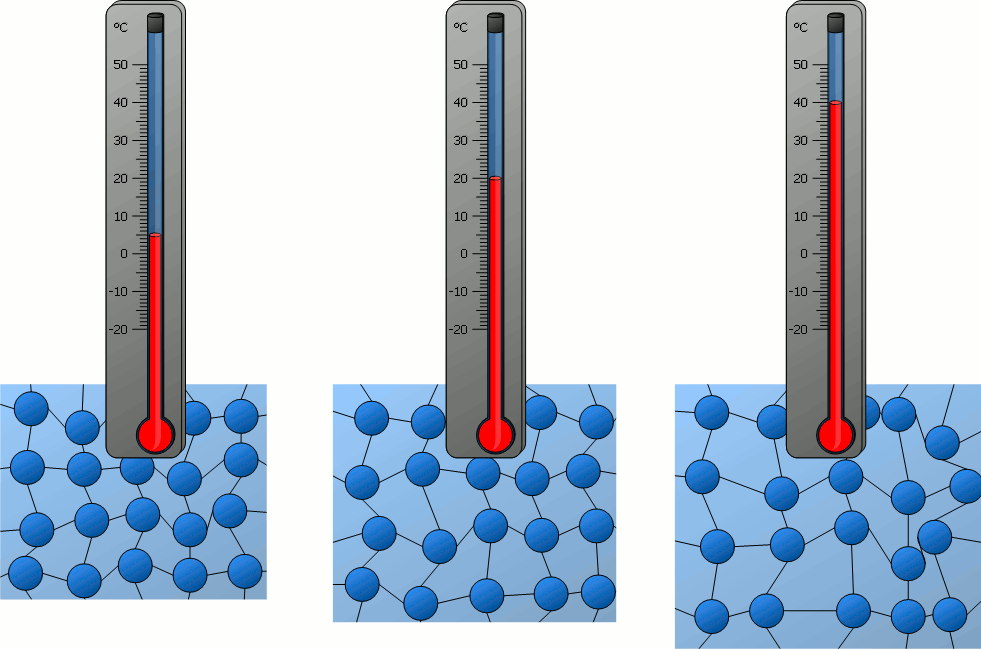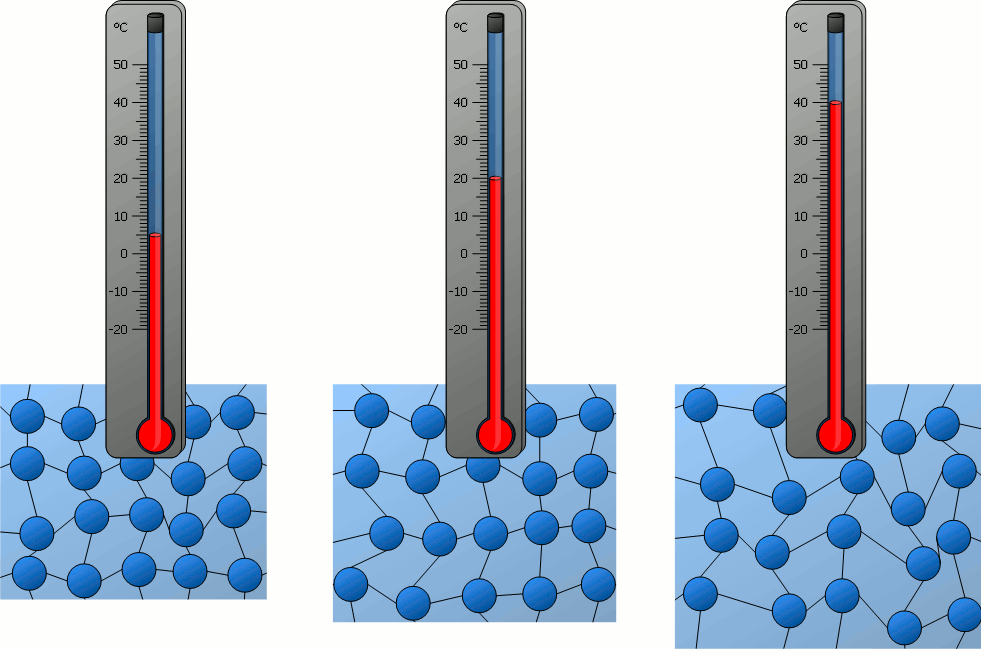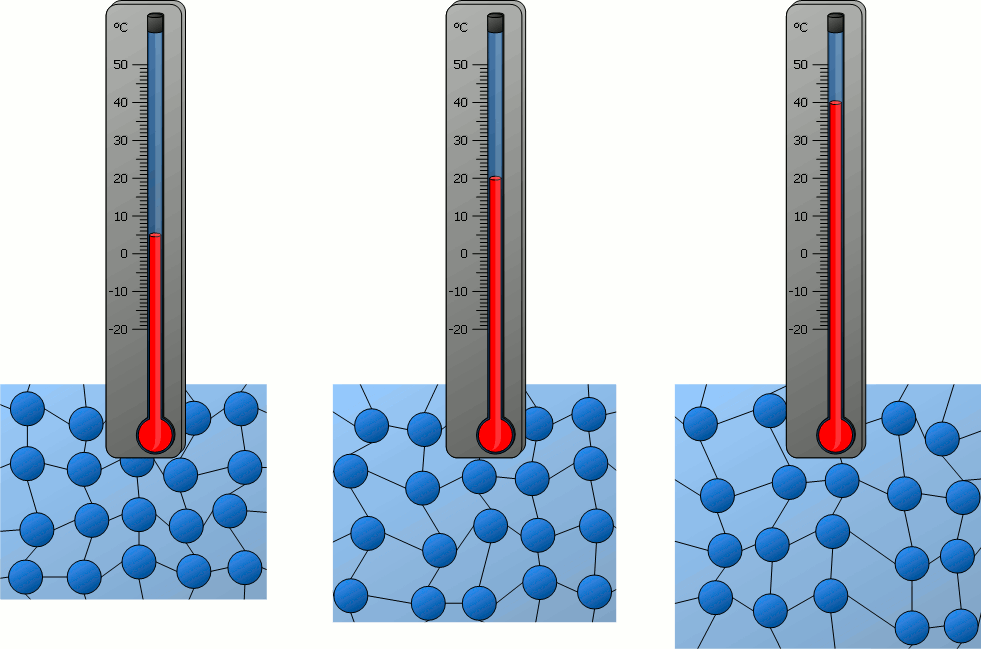
- Faster random motion of particles → higher temperature.
- At absolute zero (\(0 \, K\)), particle motion essentially ceases (minimum internal energy).
\( \overline{KE} = \dfrac{3}{2} k_B T \)
- \( \overline{KE} \): Average kinetic energy per particle
- \( k_B \): Boltzmann constant \(= 1.38 \times 10^{-23} \, \text{J/K}\)
- \( T \): Temperature in Kelvin
Maxwell–Boltzmann Distribution:
The Maxwell–Boltzmann distribution describes the spread of particle speeds in a gas at a given temperature.
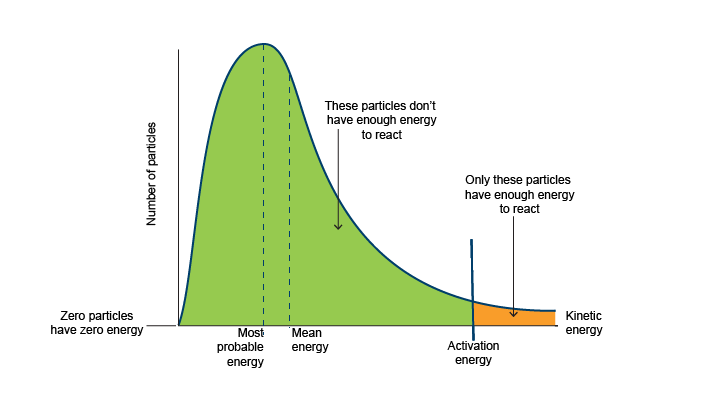
- It shows that:
- Most molecules have speeds near the most probable speed.
- Some molecules move much faster or slower.
- As temperature increases → distribution broadens, peak shifts to higher speed.
Root-Mean-Square (RMS) Speed:
The RMS speed corresponds to the average kinetic energy of molecules in an ideal gas.
\( v_{rms} = \sqrt{ \dfrac{3 k_B T}{m} } \)
- \( v_{rms} \): Root-mean-square speed of molecules
- \( m \): Mass of a single molecule
Alternative form (using molar mass \( M \)):
\( v_{rms} = \sqrt{ \dfrac{3 R T}{M} } \)
- \( R \): Gas constant \((8.314 \, \text{J/mol·K})\)
- \( M \): Molar mass of the gas (kg/mol)
Important Points:
- Temperature measures average energy, not total energy of a system.
- Different gases at the same temperature → same average kinetic energy per molecule, but different \( v_{rms} \) due to molecular mass.
- At higher temperatures → broader Maxwell–Boltzmann distribution, more high-energy particles.
Example :
Find the average kinetic energy of an oxygen molecule at \( T = 300 \, K \).
▶️ Answer/Explanation
\( \overline{KE} = \dfrac{3}{2} k_B T \)
\( = \dfrac{3}{2} (1.38 \times 10^{-23})(300) \)
\( = 6.21 \times 10^{-21} \, \text{J} \)
Answer: Each oxygen molecule has average KE ≈ \( 6.2 \times 10^{-21} \, J \).
Example :
Calculate the RMS speed of nitrogen (\( N_2 \)) at \( T = 300 \, K \). (Molar mass of \( N_2 \) = \( 0.028 \, \text{kg/mol} \)).
▶️ Answer/Explanation
\( v_{rms} = \sqrt{ \dfrac{3 R T}{M} } \)
\( = \sqrt{ \dfrac{3 (8.314)(300)}{0.028} } \)
\( = \sqrt{267,300} \)
\( \approx 517 \, \text{m/s} \)
Answer: RMS speed of nitrogen molecules ≈ \( 517 \, \text{m/s} \).
Example :
At what temperature will the RMS speed of helium (\( M = 0.004 \, \text{kg/mol} \)) be \( 1000 \, \text{m/s} \)?
▶️ Answer/Explanation
\( v_{rms} = \sqrt{ \dfrac{3 R T}{M} } \)
Rearrange: \( T = \dfrac{M v_{rms}^2}{3R} \)
\( = \dfrac{(0.004)(1000^2)}{3(8.314)} \)
\( = \dfrac{4000}{24.942} \)
\( \approx 160 \, K \)
Answer: The gas must be at ≈ \( 160 \, K \) for helium to have RMS speed of \( 1000 \, m/s \).
