AP Physics 2- 9.3 Thermal Energy Transfer and Equilibrium- Study Notes- New Syllabus
AP Physics 2- 9.3 Thermal Energy Transfer and Equilibrium – Study Notes
AP Physics 2- 9.3 Thermal Energy Transfer and Equilibrium – Study Notes – per latest Syllabus.
Key Concepts:
- Thermal Contact (Heating and Cooling)
- Modes of Energy Transfer
- Thermal Equilibrium
Thermal Contact (Heating and Cooling)
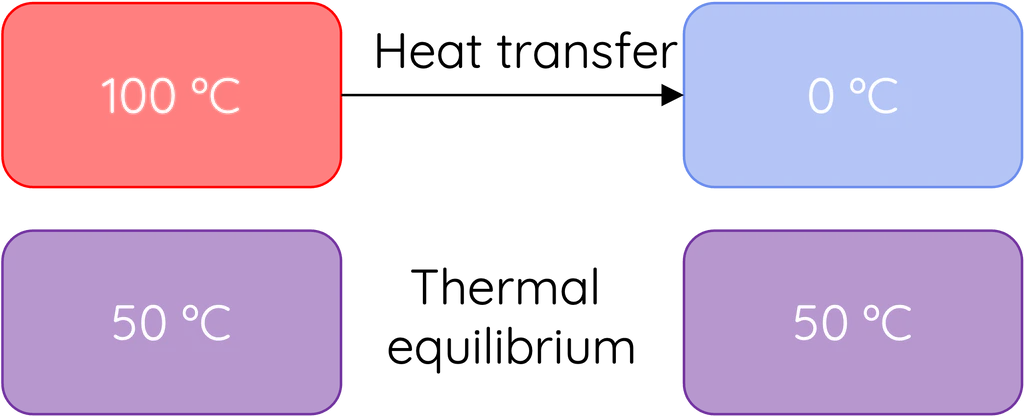
When two bodies at different temperatures are placed in thermal contact, energy is transferred between them as heat until they reach the same temperature (thermal equilibrium). The net heat transfer always occurs from the hotter object to the cooler one.
Key Concepts: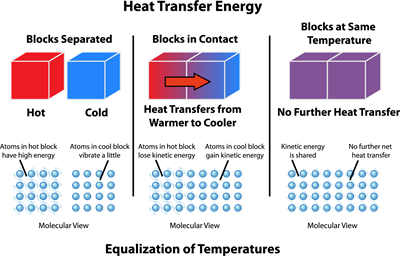
- Heat Transfer: Energy flows due to a temperature difference.
- Thermal Equilibrium: State when both bodies reach the same temperature → no net heat transfer.
- Conservation of Energy: Heat lost by the hot body = Heat gained by the cold body (assuming no losses).
Mathematical Formulation:
\( Q = mc\Delta T \)
- \( Q \): Heat absorbed/released (Joules)
- \( m \): Mass of the substance (kg)
- \( c \): Specific heat capacity (J/kg·K)
- \( \Delta T = T_{final} – T_{initial} \)
Heat Exchange Between Two Bodies:
\( m_1 c_1 (T_f – T_1) + m_2 c_2 (T_f – T_2) = 0 \)
- \( T_f \): Final equilibrium temperature
- \( T_1, T_2 \): Initial temperatures
- One term will be negative (heat lost), the other positive (heat gained).
Example :
A 0.5 kg block of aluminum (\( c = 900 \, J/kg·K \)) at 100 °C is dropped into 1 kg of water (\( c = 4200 \, J/kg·K \)) at 20 °C. Find the final equilibrium temperature (ignore heat losses).
▶️ Answer/Explanation
Using heat balance:
\( m_{Al} c_{Al} (T_f – 100) + m_{w} c_{w} (T_f – 20) = 0 \)
\( (0.5)(900)(T_f – 100) + (1)(4200)(T_f – 20) = 0 \)
\( 450(T_f – 100) + 4200(T_f – 20) = 0 \)
\( 450T_f – 45000 + 4200T_f – 84000 = 0 \)
\( 4650T_f – 129000 = 0 \)
\( T_f = \dfrac{129000}{4650} \approx 27.7 \, °C \)
Answer: Final temperature ≈ 28 °C.
Example :
200 g of copper (\( c = 390 \, J/kg·K \)) at 80 °C is placed in 100 g of water at 25 °C. Find the equilibrium temperature.
▶️ Answer/Explanation
Equation: \( m_{Cu} c_{Cu} (T_f – 80) + m_w c_w (T_f – 25) = 0 \)
\( (0.2)(390)(T_f – 80) + (0.1)(4200)(T_f – 25) = 0 \)
\( 78(T_f – 80) + 420(T_f – 25) = 0 \)
\( 78T_f – 6240 + 420T_f – 10500 = 0 \)
\( 498T_f – 16740 = 0 \)
\( T_f = \dfrac{16740}{498} \approx 33.6 \, °C \)
Answer: Final temperature ≈ 34 °C.
Modes of Energy Transfer
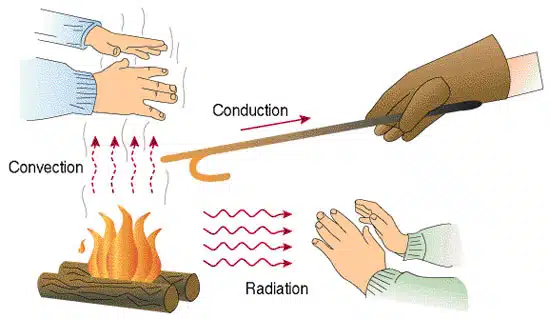
Heat can be transferred between systems or within a system by three main processes: conduction, convection, and radiation. These are the fundamental modes of thermal energy transfer.
Conduction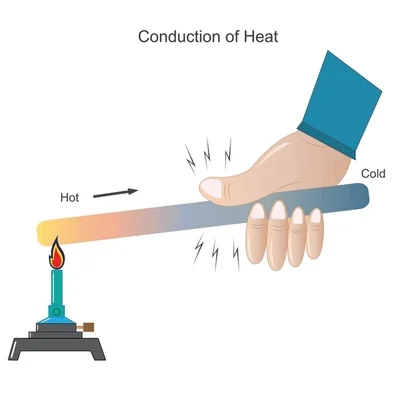
Heat transfer through a material without the bulk movement of the material itself.
- Occurs mainly in solids (especially metals) due to molecular vibrations and free electron movement.
- Fourier’s Law:
\( \dfrac{Q}{t} = \dfrac{kA (T_{hot} – T_{cold})}{L} \)
- \( Q/t \): Rate of heat transfer (W)
- \( k \): Thermal conductivity (W/m·K)
- \( A \): Cross-sectional area (m²)
- \( L \): Thickness (m)
Convection
Heat transfer due to the bulk movement of fluid (liquid or gas).
- Hot fluid rises, cold fluid sinks → circulation transfers energy.
- Two types:
- Natural convection: Driven by density differences (buoyancy).
- Forced convection: Driven by external agents like fans or pumps.
- Equation (approximate):
\( \dfrac{Q}{t} = hA (T_s – T_f) \)
- \( h \): Convection heat transfer coefficient (W/m²·K)
- \( T_s \): Surface temperature
- \( T_f \): Fluid temperature
Radiation
Heat transfer by electromagnetic waves (infrared radiation).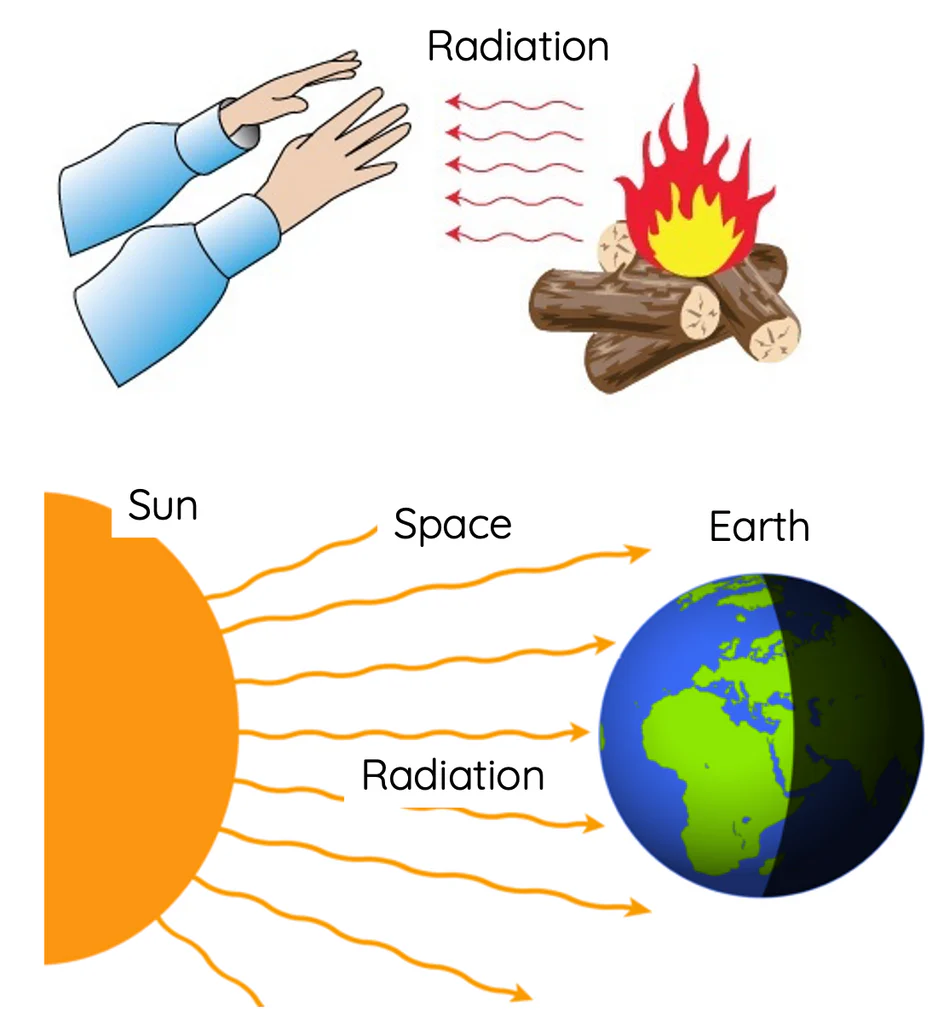
- No medium required (can occur in vacuum).
Stefan–Boltzmann Law:
\( P = \epsilon \sigma A T^4 \)
- \( P \): Radiated power (W)
- \( \epsilon \): Emissivity (0–1)
- \( \sigma \): Stefan–Boltzmann constant \( = 5.67 \times 10^{-8} \, W/m^2K^4 \)
- \( A \): Surface area (m²)
- \( T \): Absolute temperature (K)
Example :
A metal rod of length 0.5 m and cross-sectional area \( 2 \times 10^{-4} \, m^2 \) has thermal conductivity \( k = 200 \, W/m·K \). One end is kept at 100 °C and the other at 0 °C. Find the rate of heat transfer by conduction.
▶️ Answer/Explanation
Using Fourier’s law:
\( \dfrac{Q}{t} = \dfrac{kA (T_{hot} – T_{cold})}{L} \)
\( = \dfrac{(200)(2 \times 10^{-4})(100 – 0)}{0.5} \)
\( = \dfrac{4}{0.5} = 8 \, W \)
Answer: Heat transfer rate = 8 W.
Example :
A hot surface of area \( 0.1 \, m^2 \) at 80 °C loses heat to surrounding air at 30 °C by convection. If the convection coefficient is \( h = 25 \, W/m^2K \), find the heat loss rate.
▶️ Answer/Explanation
\( \dfrac{Q}{t} = hA (T_s – T_f) \)
\( = (25)(0.1)(80 – 30) \)
\( = 25 \times 0.1 \times 50 \)
\( = 125 \, W \)
Answer: Heat loss = 125 W.
Example :
A body of surface area \( 0.5 \, m^2 \), emissivity 0.8, and temperature 500 K radiates energy. Find the radiated power.
▶️ Answer/Explanation
\( P = \epsilon \sigma A T^4 \)
\( = (0.8)(5.67 \times 10^{-8})(0.5)(500^4) \)
\( = (0.8)(5.67 \times 10^{-8})(0.5)(6.25 \times 10^{10}) \)
\( \approx 1416 \, W \)
Answer: Radiated power ≈ 1.4 kW.
Thermal Equilibrium
Thermal equilibrium is the state in which two or more bodies in thermal contact exchange no net heat because they are at the same temperature.
Key Points:
No Net Heat Flow: When objects reach the same temperature, heat transfer stops.
Consequence of Zeroth Law of Thermodynamics:
- If body A is in thermal equilibrium with body B, and body B is in equilibrium with body C, then A and C are also in equilibrium.

Energy Conservation: In a closed system, heat lost by hot objects = heat gained by cold objects until equilibrium temperature is reached.
Equilibrium Temperature (\(T_f\)): Can be calculated using the principle of conservation of heat:
\( \sum Q = 0 \)
Mathematical Expression:
\( m_1 c_1 (T_f – T_1) + m_2 c_2 (T_f – T_2) + \dots = 0 \)
- \( m \): Mass
- \( c \): Specific heat capacity
- \( T_f \): Final equilibrium temperature
- \( T \): Initial temperature
Example :
A 0.2 kg piece of iron (\( c = 450 \, J/kg·K \)) at 200 °C is placed in 0.5 kg of water at 25 °C (\( c = 4200 \, J/kg·K \)). Find the equilibrium temperature. (Ignore heat loss to surroundings.)
▶️ Answer/Explanation
Heat lost by iron = Heat gained by water
\( m_{Fe} c_{Fe} (T_f – 200) + m_w c_w (T_f – 25) = 0 \)
\( (0.2)(450)(T_f – 200) + (0.5)(4200)(T_f – 25) = 0 \)
\( 90(T_f – 200) + 2100(T_f – 25) = 0 \)
\( 90T_f – 18000 + 2100T_f – 52500 = 0 \)
\( 2190T_f – 70500 = 0 \)
\( T_f = \dfrac{70500}{2190} \approx 32.2 \, °C \)
Answer: Final equilibrium temperature ≈ 32 °C.
Example :
Two objects, A and B, are placed in thermal contact. A has heat capacity \( C_A = 500 \, J/K \) and is initially at 350 K, while B has \( C_B = 300 \, J/K \) and is initially at 250 K. Find their equilibrium temperature.
▶️ Answer/Explanation
Conservation of heat: \( C_A (T_f – 350) + C_B (T_f – 250) = 0 \)
\( 500(T_f – 350) + 300(T_f – 250) = 0 \)
\( 500T_f – 175000 + 300T_f – 75000 = 0 \)
\( 800T_f – 250000 = 0 \)
\( T_f = \dfrac{250000}{800} = 312.5 \, K \)
Answer: Equilibrium temperature = 312.5 K.