AP Physics 2- 9.4 The First Law of Thermodynamics- Study Notes- New Syllabus
AP Physics 2- 9.4 The First Law of Thermodynamics – Study Notes
AP Physics 2- 9.4 The First Law of Thermodynamics – Study Notes – per latest Syllabus.
Key Concepts:
- Internal Energy of a System
- Behavior of a System Using Thermodynamic Processes
Internal Energy of a System
Internal energy (\(U\)) of a system is the total energy contained within the system due to the microscopic motion and interactions of its particles. It includes both the kinetic energy of particles (due to their motion) and the potential energy (due to intermolecular forces).
Key Concepts:
- Kinetic Energy Contribution: Energy from the random motion of molecules, atoms, or ions. For ideal gases, this depends only on temperature.
- Potential Energy Contribution: Energy due to intermolecular forces, important in liquids and solids.
- State Function: Internal energy depends only on the current state of the system (temperature, pressure, volume), not on how the system reached that state.
Change in Internal Energy (\(\Delta U\)): Can occur due to heat transfer (\(Q\)) or work done (\(W\)) on/by the system:
\( \Delta U = Q – W \)
For Ideal Gas: Internal energy depends only on temperature:
\( U = \dfrac{3}{2} n R T \) (monatomic gas)
\( U = \dfrac{5}{2} n R T \) (diatomic gas at moderate temperatures)
Example :
Find the internal energy of 2 moles of a monatomic ideal gas at 300 K.
▶️ Answer/Explanation
\( U = \dfrac{3}{2} n R T \)
\( = \dfrac{3}{2} (2)(8.314)(300) \)
\( = 7476 \, J \)
Answer: Internal energy ≈ 7.48 kJ.
Example :
The internal energy of 1 mole of a diatomic ideal gas increases from 300 K to 600 K. Find the change in internal energy. (Take \(U = \dfrac{5}{2} n R T\))
▶️ Answer/Explanation
\( \Delta U = \dfrac{5}{2} n R (T_2 – T_1) \)
\( = \dfrac{5}{2} (1)(8.314)(600 – 300) \)
\( = \dfrac{5}{2} (8.314)(300) \)
\( = 6235.5 \, J \)
Answer: Change in internal energy ≈ 6.24 kJ.
Behavior of a System Using Thermodynamic Processes
A thermodynamic process describes how a system changes its state (pressure, volume, temperature, internal energy) due to heat transfer or work done. By analyzing these processes, we can understand how energy is exchanged within the system.
Key Concepts:
State Variables: Pressure (\(P\)), Volume (\(V\)), Temperature (\(T\)), and Internal Energy (\(U\)).
Work Done by the System:
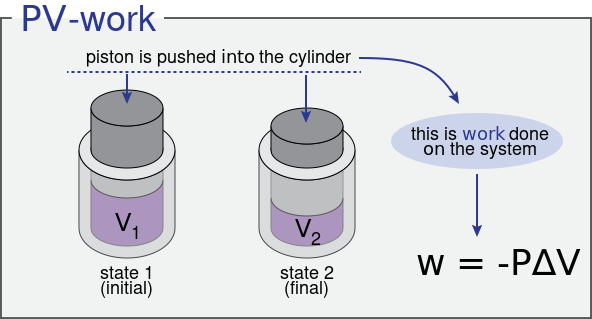
\( W = \int P \, dV \)
- Positive if the system expands (does work on surroundings).
- Negative if the system is compressed (work done on the system).
Heat Transfer (\(Q\)): Energy added to or removed from the system.
First Law of Thermodynamics:
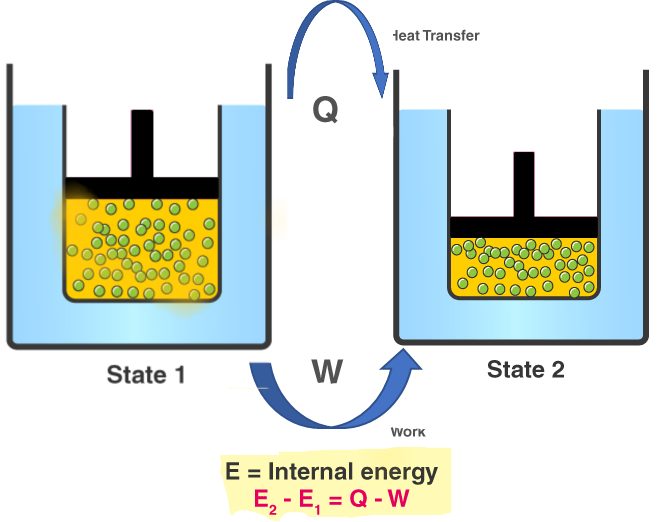
\( \Delta U = Q – W \)
Relates change in internal energy, heat added, and work done.
Common Thermodynamic Processes:
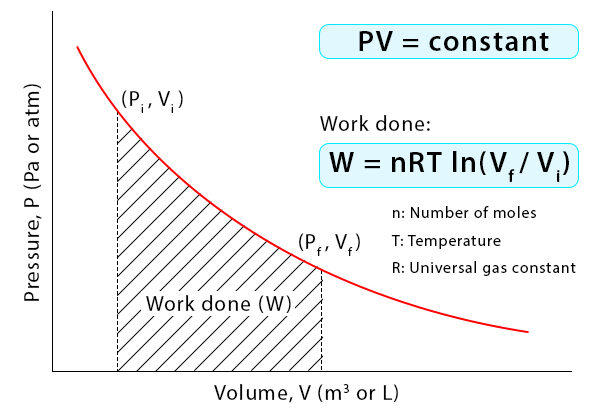
Isothermal Process (\(T = \text{constant}\))

- Temperature remains constant → \(\Delta U = 0\)
- Heat added equals work done by the system: \( Q = W \)
- PV relation: \( PV = \text{constant} \)
Isochoric / Isometric Process (\(V = \text{constant}\))
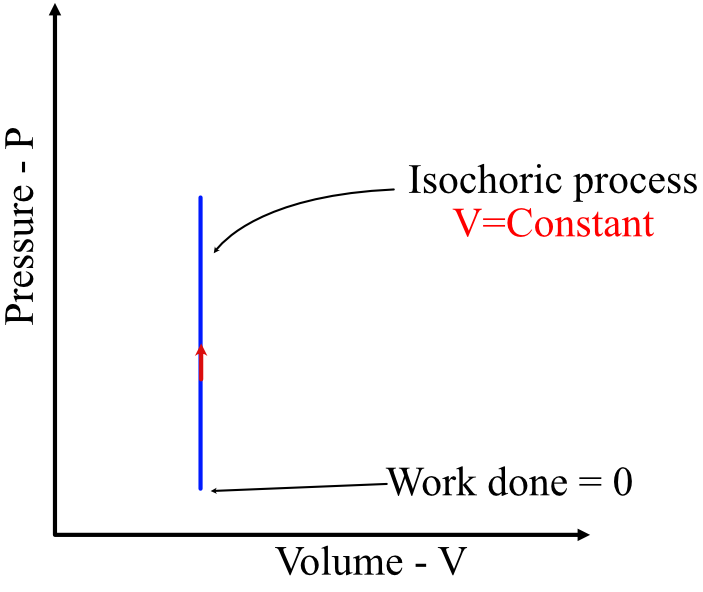
- Volume is constant → no work is done: \( W = 0 \)
- Heat transfer changes internal energy: \( Q = \Delta U \)
Isobaric Process (\(P = \text{constant}\))
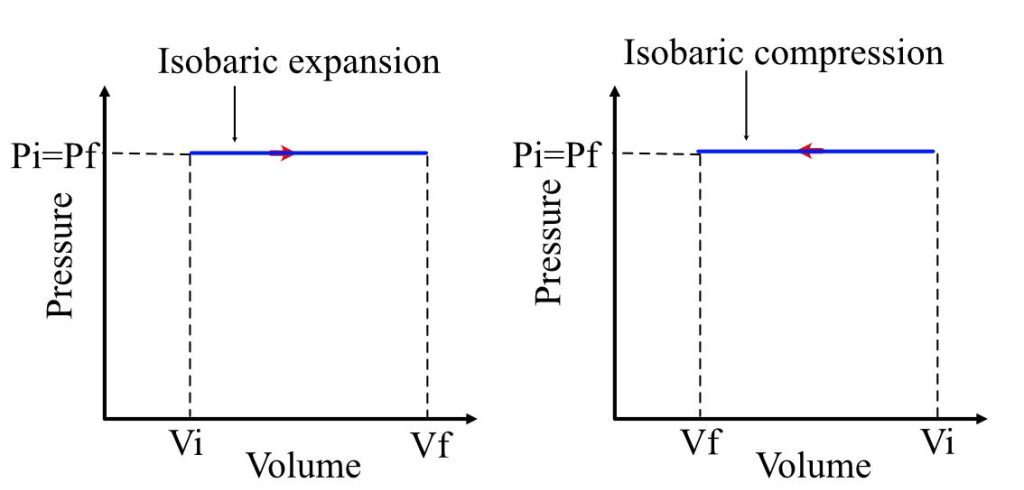
- Pressure remains constant
- Work done: \( W = P \Delta V \)
- Heat transfer affects both internal energy and work: \( Q = \Delta U + P \Delta V \)
Adiabatic Process (\(Q = 0\))
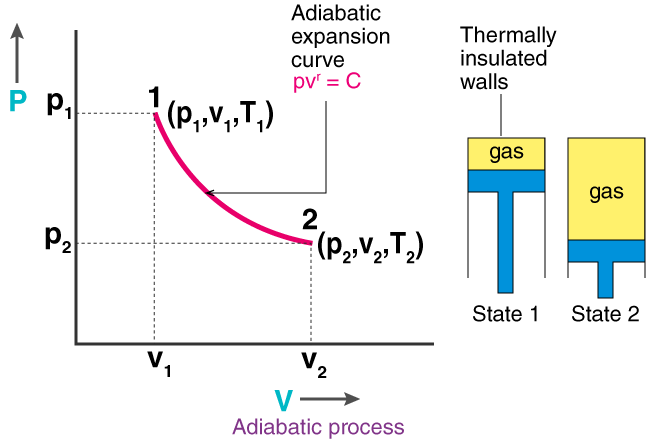
- No heat exchange with surroundings
- Work done changes internal energy: \( \Delta U = -W \)
- PV relation: \( PV^\gamma = \text{constant} \), where \( \gamma = C_p/C_v \)
Graphical Relation Summary:
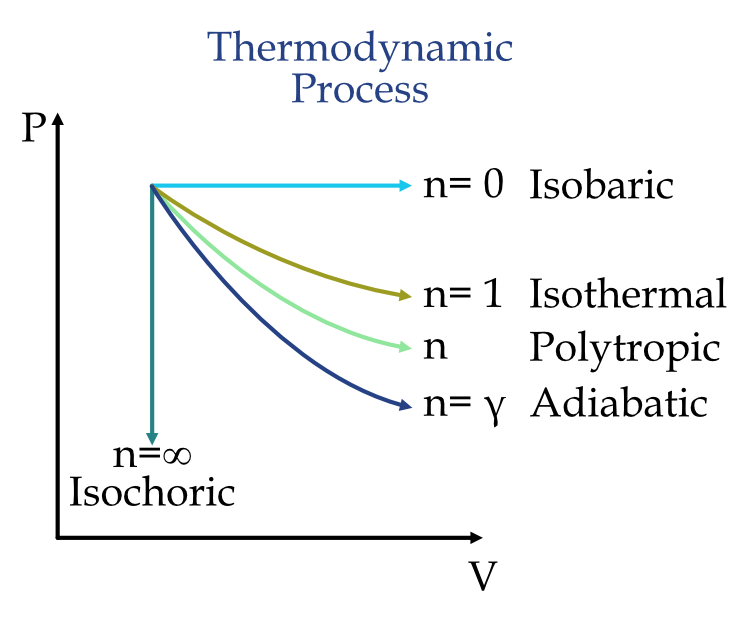
Example :
1 mole of an ideal gas expands isothermally at 300 K from 2 L to 4 L. Find the work done.
▶️ Answer/Explanation
For isothermal process: \( W = nRT \ln \dfrac{V_f}{V_i} \)
\( W = (1)(8.314)(300) \ln \dfrac{4}{2} \)
\( = 2494.2 \ln 2 \approx 2494.2 \times 0.693 \approx 1727 \, J \)
Answer: Work done ≈ 1.73 kJ.
Example :
A gas in a cylinder is heated at constant volume from 300 K to 600 K. Mass of gas = 2 moles, \( C_v = 12.5 \, J/mol·K \). Find \( \Delta U \).
▶️ Answer/Explanation
For isochoric process: \( \Delta U = n C_v \Delta T \)
\( \Delta U = 2 \times 12.5 \times (600 – 300) = 2 \times 12.5 \times 300 = 7500 \, J \)
Answer: Change in internal energy = 7.5 kJ.
